Abstract
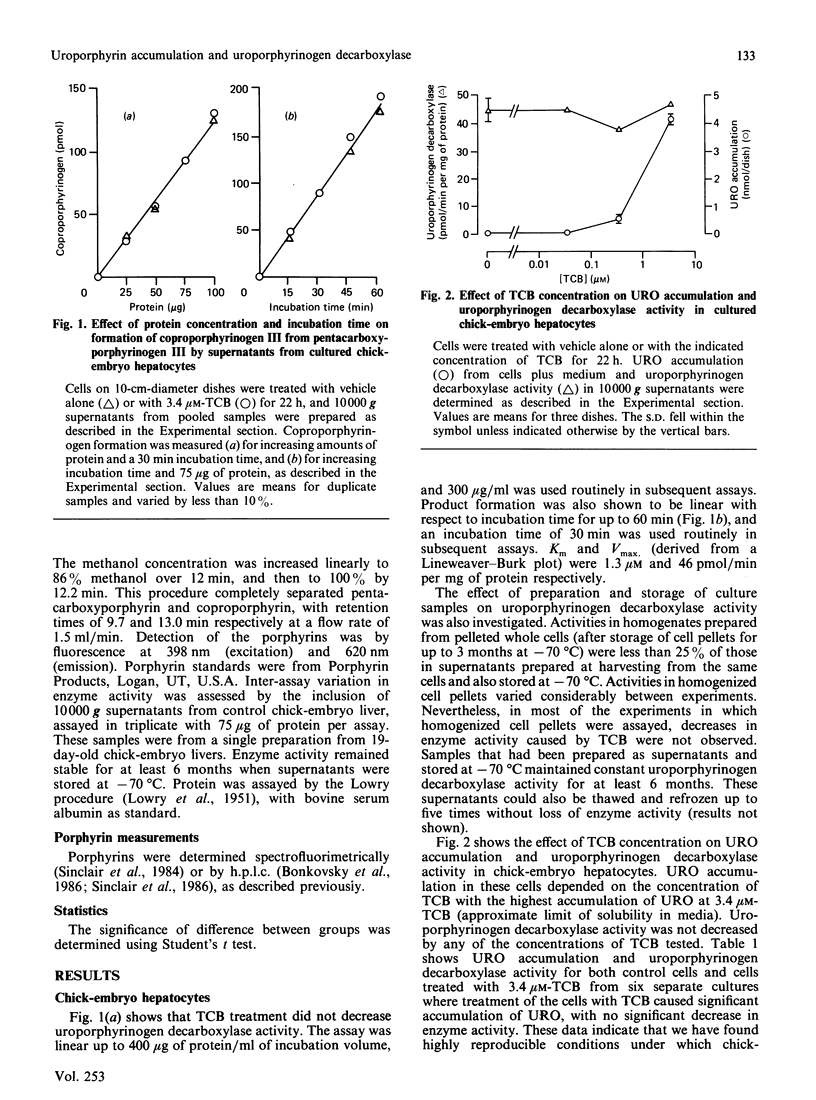
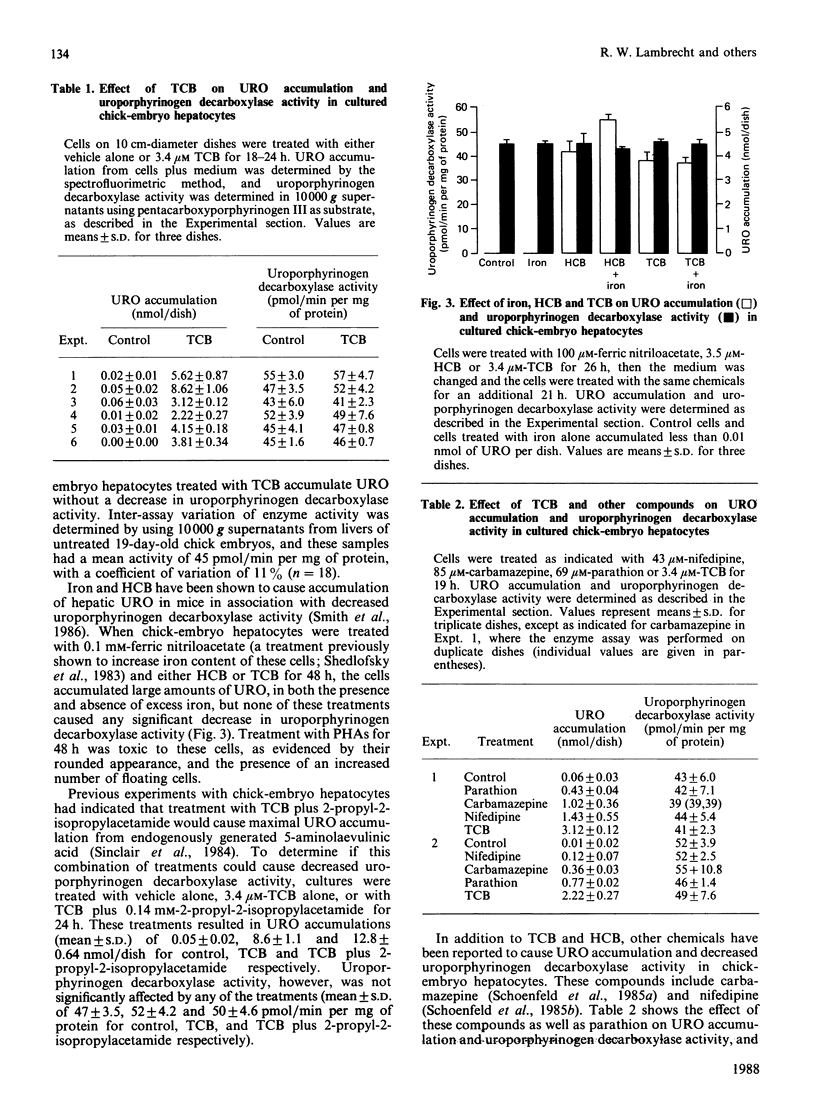
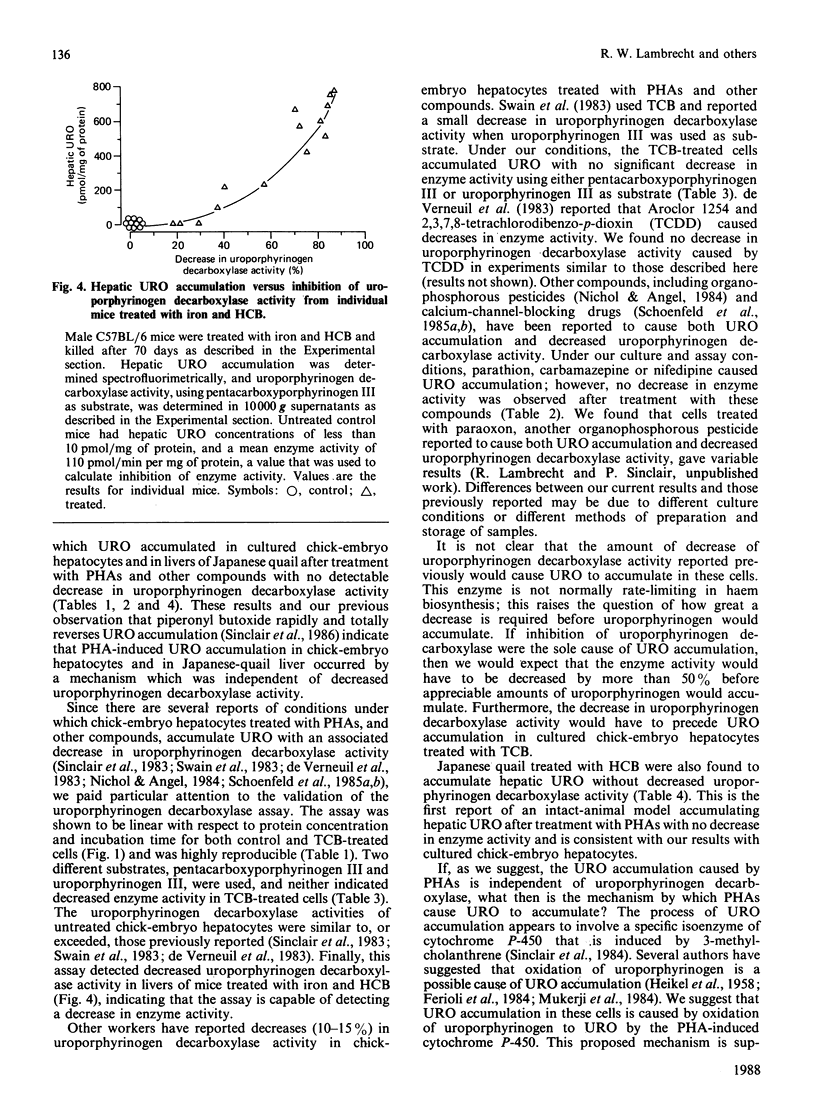
The relationship between hepatic uroporphyrin accumulation and uroporphyrinogen decarboxylase (EC 4.1.1.37) activity was investigated in cultured chick-embryo hepatocytes, Japanese quail (Coturnix coturnix japonica) and mice that had been treated with polyhalogenated aromatic compounds. Chick-embryo hepatocytes treated with 3,3',4,4'-tetrachlorobiphenyl accumulated uroporphyrin in a dose-dependent fashion without a detectable decrease in uroporphyrinogen decarboxylase activity when either pentacarboxyporphyrinogen III or uroporphyrinogen III were used as substrates in the assay. Other compounds, such as hexachlorobenzene, parathion, carbamazepine and nifedipine, which have been shown previously to cause uroporphyrin accumulation in these cells, did not decrease uroporphyrinogen decarboxylase activity. Japanese quail treated with hexachlorobenzene for 7-10 days also accumulated hepatic uroporphyrin without any decrease in uroporphyrinogen decarboxylase activity. In contrast, hepatic uroporphyrin accumulation in male C57BL/6 mice treated with iron and hexachlorobenzene was accompanied by a 20-80% decrease in uroporphyrinogen decarboxylase activity, demonstrating that the assay used for uroporphyrinogen decarboxylase, using pentacarboxyporphyrinogen III as substrate, could detect decreased enzyme activity. Our results with chick hepatocytes and quail, showing uroporphyrin accumulation without a decrease in uroporphyrinogen decarboxylase activity, are consistent with a new two-stage model of the uroporphyria: initially uroporphyrinogen is oxidized by a cytochrome P-450-mediated reaction, followed in rodents by a progressive decrease in uroporphyrinogen decarboxylase activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonkovsky H. L., Wood S. G., Howell S. K., Sinclair P. R., Lincoln B., Healey J. F., Sinclair J. F. High-performance liquid chromatographic separation and quantitation of tetrapyrroles from biological materials. Anal Biochem. 1986 May 15;155(1):56–64. doi: 10.1016/0003-2697(86)90224-1. [DOI] [PubMed] [Google Scholar]
- Cantoni L., Ruggieri R., Dal Fiume D., Rizzardini M. The determination of uroporphyrinogen decarboxylase in tissues by high-performance liquid chromatography coupled to fluorescence detection. J Chromatogr. 1982 May 14;229(2):311–318. doi: 10.1016/s0378-4347(00)84273-5. [DOI] [PubMed] [Google Scholar]
- Cantoni L., dal Fiume D., Rizzardini M., Ruggieri R. In vitro inhibitory effect on porphyrinogen carboxylyase of liver extracts from TCDD treated mice. Toxicol Lett. 1984 Feb;20(2):211–217. doi: 10.1016/0378-4274(84)90149-8. [DOI] [PubMed] [Google Scholar]
- Carpenter H. M., Williams D. E., Buhler D. R. Hexachlorobenzene-induced porphyria in Japanese quail: changes in microsomal enzymes. J Toxicol Environ Health. 1985;15(3-4):431–444. doi: 10.1080/15287398509530670. [DOI] [PubMed] [Google Scholar]
- DE MATTEIS F., PRIOR B. E., RIMINGTON C. Nervous and biochemical disturbances following hexachlorobenzene intoxication. Nature. 1961 Jul 22;191:363–366. doi: 10.1038/191363a0. [DOI] [PubMed] [Google Scholar]
- De Matteis F., Harvey C., Reed C., Hempenius R. Increased oxidation of uroporphyrinogen by an inducible liver microsomal system. Possible relevance to drug-induced uroporphyria. Biochem J. 1988 Feb 15;250(1):161–169. doi: 10.1042/bj2500161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Verneuil H., Sassa S., Kappas A. Effects of polychlorinated biphenyl compounds, 2,3,7,8-tetrachlorodibenzo-p-dioxin, phenobarbital and iron on hepatic uroporphyrinogen decarboxylase. Implications for the pathogenesis of porphyria. Biochem J. 1983 Jul 15;214(1):145–151. doi: 10.1042/bj2140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder G. H., Evans J. O., Matlin S. A. The effect of the porphyrogenic compound, hexachlorobenzene, on the activity of hepatic uroporphyrinogen decarboxylase in the rat. Clin Sci Mol Med. 1976 Jul;51(1):71–80. doi: 10.1042/cs0510071. [DOI] [PubMed] [Google Scholar]
- Elder G. H., Wyvill P. C. Measurement of uroporphyrinogen decarboxylase using porphyrinogens prepared by chemical reduction. Enzyme. 1982;28(2-3):186–195. doi: 10.1159/000459101. [DOI] [PubMed] [Google Scholar]
- Ferioli A., Harvey C., De Matteis F. Drug-induced accumulation of uroporphyrin in chicken hepatocyte cultures. Structural requirements for the effect and role of exogenous iron. Biochem J. 1984 Dec 15;224(3):769–777. doi: 10.1042/bj2240769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis J. E., Smith A. G. Assay of mouse liver uroporphyrinogen decarboxylase by reverse-phase high-performance liquid chromatography. Anal Biochem. 1984 May 1;138(2):404–410. doi: 10.1016/0003-2697(84)90829-7. [DOI] [PubMed] [Google Scholar]
- Gocmen A., Peters H. A., Cripps D. J., Morris C. R., Dogramaci I. Porphyria turcica: hexachlorobenzene-induced porphyria. IARC Sci Publ. 1986;(77):567–573. [PubMed] [Google Scholar]
- Goldstein J. A., Hickman P., Bergman H., Vos J. G. Hepatic porphyria induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in the mouse. Res Commun Chem Pathol Pharmacol. 1973 Nov;6(3):919–928. [PubMed] [Google Scholar]
- HEIKEL T., LOCKWOOD W. H., RIMINGTON C. Formation of non-enzymic haem. Nature. 1958 Aug 2;182(4631):313–313. doi: 10.1038/182313a0. [DOI] [PubMed] [Google Scholar]
- Jones K. G., Sweeney G. D. Dependence of the porphyrogenic effect of 2,3,7,8-tetrachlorodibenzo(p)dioxin upon inheritance of aryl hydrocarbon hydroxylase responsiveness. Toxicol Appl Pharmacol. 1980 Mar 30;53(1):42–49. doi: 10.1016/0041-008x(80)90379-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lyon M. E., Owen J. A., Marks G. S. Xenobiotic mediated inhibition of hepatic uroporphyrinogen decarboxylase activity in 17-day-old chick embryo liver cells in culture. Biochem Pharmacol. 1988 Mar 15;37(6):1123–1129. doi: 10.1016/0006-2952(88)90520-5. [DOI] [PubMed] [Google Scholar]
- Marks G. S. Exposure to toxic agents: the heme biosynthetic pathway and hemoproteins as indicator. Crit Rev Toxicol. 1985;15(2):151–179. doi: 10.3109/10408448509029323. [DOI] [PubMed] [Google Scholar]
- Mukerji S. K., Pimstone N. R., Burns M. Dual mechanism of inhibition of rat liver uroporphyrinogen decarboxylase activity by ferrous iron: its potential role in the genesis of porphyria cutanea tarda. Gastroenterology. 1984 Dec;87(6):1248–1254. [PubMed] [Google Scholar]
- Nichol A. W., Angel L. A. A comparative study of porphyrin accumulation in tissue cultures of chicken embryo hepatocytes treated with organophosphorous pesticides. Biochem Pharmacol. 1984 Aug 1;33(15):2511–2515. doi: 10.1016/0006-2952(84)90726-3. [DOI] [PubMed] [Google Scholar]
- Schoenfeld N., Aelion J., Beigel Y., Epstein O., Atsmon A. The porphyrogenic effects of calcium channel blocking drugs. Clin Sci (Lond) 1985 Nov;69(5):581–586. doi: 10.1042/cs0690581. [DOI] [PubMed] [Google Scholar]
- Schoenfeld N., Greenblat Y., Epstein O., Atsmon A. The influence of carbamazepine on the heme biosynthetic pathway. Biochem Med. 1985 Dec;34(3):280–286. doi: 10.1016/0006-2944(85)90089-4. [DOI] [PubMed] [Google Scholar]
- Shedlofsky S. I., Bonkowsky H. L., Sinclair P. R., Sinclair J. F., Bement W. J., Pomeroy J. S. Iron loading of cultured hepatocytes. Effect of iron on 5-aminolaevulinate synthase is independent of lipid peroxidation. Biochem J. 1983 May 15;212(2):321–330. doi: 10.1042/bj2120321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair J. F., Sinclair P. R., Healey J. F., Smith E. L., Bonkowsky H. L. Decrease in hepatic cytochrome P-450 by cobalt. Evidence for a role of cobalt protoporphyrin. Biochem J. 1982 Apr 15;204(1):103–109. doi: 10.1042/bj2040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair P. R., Bement W. J., Bonkovsky H. L., Lambrecht R. W., Frezza J. E., Sinclair J. F., Urquhart A. J., Elder G. H. Uroporphyrin accumulation produced by halogenated biphenyls in chick-embryo hepatocytes. Reversal of the accumulation by piperonyl butoxide. Biochem J. 1986 Jul 1;237(1):63–71. doi: 10.1042/bj2370063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair P. R., Bement W. J., Bonkovsky H. L., Sinclair J. F. Inhibition of uroporphyrinogen decarboxylase by halogenated biphenyls in chick hepatocyte cultures. Essential role for induction of cytochrome P-448. Biochem J. 1984 Sep 15;222(3):737–748. doi: 10.1042/bj2220737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair P. R., Elder G. H., Bement W. J., Smith S. G., Bonkowsky H. L., Sinclair J. F. Decreased activity of uroporphyrinogen decarboxylase caused by 2,4,5,3',4'-pentabromobiphenyl in chick embryo hepatocyte cultures. Difference in activity in intact or homogenized cells. FEBS Lett. 1983 Feb 21;152(2):217–221. doi: 10.1016/0014-5793(83)80383-4. [DOI] [PubMed] [Google Scholar]
- Sinclair P. R., Granick S. Uroporphyrin formation induced by chlorinated hydrocarbons (lindane, polychlorinated biphenyls, tetrachlorodibenzo-p-dioxin). Requirements for endogenous iron, protein synthesis and drug-metabolizing activity. Biochem Biophys Res Commun. 1974 Nov 6;61(1):124–133. doi: 10.1016/0006-291x(74)90543-9. [DOI] [PubMed] [Google Scholar]
- Sinclair P., Lambrecht R., Sinclair J. Evidence for cytochrome P450-mediated oxidation of uroporphyrinogen by cell-free liver extracts from chick embryos treated with 3-methylcholanthrene. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1324–1329. doi: 10.1016/0006-291x(87)90794-7. [DOI] [PubMed] [Google Scholar]
- Smith A. G., Francis J. E. Chemically-induced formation of an inhibitor of hepatic uroporphyrinogen decarboxylase in inbred mice with iron overload. Biochem J. 1987 Aug 15;246(1):221–226. doi: 10.1042/bj2460221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. G., Francis J. E., Kay S. J., Greig J. B., Stewart F. P. Mechanistic studies of the inhibition of hepatic uroporphyrinogen decarboxylase in C57BL/10 mice by iron-hexachlorobenzene synergism. Biochem J. 1986 Sep 15;238(3):871–878. doi: 10.1042/bj2380871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. G., Francis J. E. Synergism of iron and hexachlorobenzene inhibits hepatic uroporphyrinogen decarboxylase in inbred mice. Biochem J. 1983 Sep 15;214(3):909–913. doi: 10.1042/bj2140909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain M. G., Follows S. B., Marks G. S. Inhibition of uroporphyrinogen decarboxylase by 3,3',4,4'-tetrachlorobiphenyl in chick embryo liver cell culture. Can J Physiol Pharmacol. 1983 Jan;61(1):105–108. doi: 10.1139/y83-014. [DOI] [PubMed] [Google Scholar]
- Voorman R., Aust S. D. Specific binding of polyhalogenated aromatic hydrocarbon inducers of cytochrome P-450d to the cytochrome and inhibition of its estradiol 2-hydroxylase activity. Toxicol Appl Pharmacol. 1987 Aug;90(1):69–78. doi: 10.1016/0041-008x(87)90307-3. [DOI] [PubMed] [Google Scholar]
- Vos J. G., Strik J. J., van Holsteÿn C. W., Pennings J. H. Polychlorinated biphenyls as inducers of hepatic porphyria in Japanese quail, with special reference to -aminolevulinic acid synthetase activity, fluorescence, and residues in the liver. Toxicol Appl Pharmacol. 1971 Oct;20(2):232–240. doi: 10.1016/0041-008x(71)90049-4. [DOI] [PubMed] [Google Scholar]
- de Verneuil H., Grandchamp B., Nordmann Y. Some kinetic properties of human red cell uroporphyrinogen decarboxylase. Biochim Biophys Acta. 1980 Jan 11;611(1):174–186. doi: 10.1016/0005-2744(80)90053-4. [DOI] [PubMed] [Google Scholar]


