Abstract
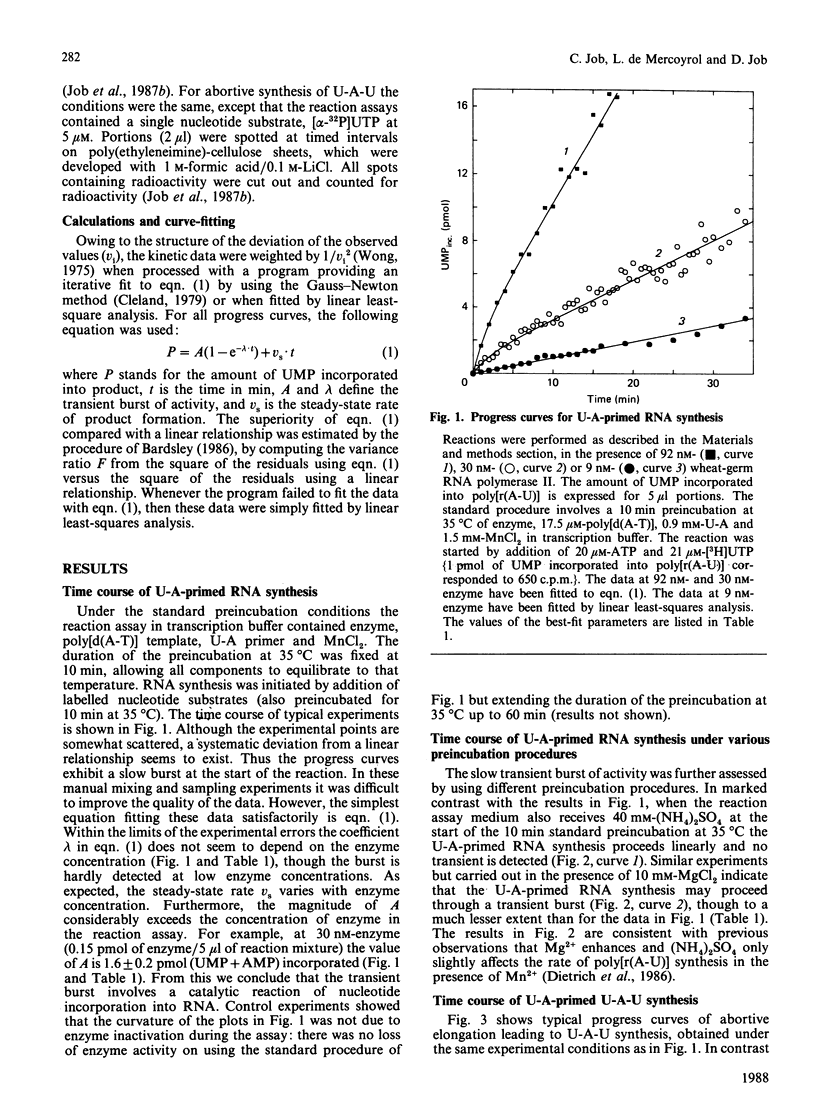
Progress curves of U-A-primed RNA synthesis catalysed by wheat-germ RNA polymerase II on a poly[d(A-T)] template exhibit a slow burst of activity. In contrast, the progress curves of single-step addition of UMP to U-A primer in the abortive elongation reaction do not exhibit the slow burst of activity. The correlation between the kinetic transient in the productive pathway of RNA synthesis and the rate of abortive elongation is suggestive of the occurrence of a slow conformational change of the transcription complex during the transition from abortive to productive elongation. The exceptional duration of the transient burst (in the region of 4 min) may suggest a transition of a hysteretic type.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armaleo D. Structure and evolution of prokaryotic and eukaryotic RNA polymerases: a model. J Theor Biol. 1987 Aug 7;127(3):301–314. doi: 10.1016/s0022-5193(87)80108-x. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A., Cárdenas M. L. Co-operativity in monomeric enzymes. J Theor Biol. 1987 Jan 7;124(1):1–23. doi: 10.1016/s0022-5193(87)80248-5. [DOI] [PubMed] [Google Scholar]
- Dietrich J., Teissere M., Job C., Job D. Effect of salts on abortive and productive elongation catalysed by wheat germ RNA polymerase II. Nucleic Acids Res. 1986 Feb 25;14(4):1583–1597. doi: 10.1093/nar/14.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J., Teissere M., Job C., Job D. Poly(dAT) dependent trinucleotide synthesis catalysed by wheat germ RNA polymerase II. Effects of nucleotide substrates and cordycepin triphosphate. Nucleic Acids Res. 1985 Sep 11;13(17):6155–6170. doi: 10.1093/nar/13.17.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieden C. Kinetic aspects of regulation of metabolic processes. The hysteretic enzyme concept. J Biol Chem. 1970 Nov 10;245(21):5788–5799. [PubMed] [Google Scholar]
- Jendrisak J. J., Burgess R. R. A new method for the large-scale purification of wheat germ DNA-dependent RNA polymerase II. Biochemistry. 1975 Oct 21;14(21):4639–4645. doi: 10.1021/bi00692a012. [DOI] [PubMed] [Google Scholar]
- Job C., Briat J. F., Lescure A. M., Job D. Abortive and productive elongation catalysed by purified spinach chloroplast RNA polymerase. Eur J Biochem. 1987 Jun 15;165(3):515–519. doi: 10.1111/j.1432-1033.1987.tb11469.x. [DOI] [PubMed] [Google Scholar]
- Job C., Dietrich J., Shire D., Teissere M., Job D. Effect of low nucleotide concentrations on abortive elongation catalysed by wheat-germ RNA polymerase II. Biochem J. 1987 May 15;244(1):151–157. doi: 10.1042/bj2440151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job C., Soulié J. M., Job D. Kinetic co-operativity of wheat-germ RNA polymerase II with adenosine 5'-[beta gamma-imido]triphosphate as substrate. Biochem J. 1988 May 15;252(1):55–63. doi: 10.1042/bj2520055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchta R. D., Mizrahi V., Benkovic P. A., Johnson K. A., Benkovic S. J. Kinetic mechanism of DNA polymerase I (Klenow). Biochemistry. 1987 Dec 15;26(25):8410–8417. doi: 10.1021/bi00399a057. [DOI] [PubMed] [Google Scholar]
- Lescure B., Williamson V., Sentenac A. Efficient and selective initiation by yeast RNA polymerase B in a dinucleotide-primed reaction. Nucleic Acids Res. 1981 Jan 10;9(1):31–45. doi: 10.1093/nar/9.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luse D. S., Jacob G. A. Abortive initiation by RNA polymerase II in vitro at the adenovirus 2 major late promoter. J Biol Chem. 1987 Nov 5;262(31):14990–14997. [PubMed] [Google Scholar]
- Luse D. S., Kochel T., Kuempel E. D., Coppola J. A., Cai H. Transcription initiation by RNA polymerase II in vitro. At least two nucleotides must be added to form a stable ternary complex. J Biol Chem. 1987 Jan 5;262(1):289–297. [PubMed] [Google Scholar]
- Neet K. E., Ainslie G. R., Jr Hysteretic enzymes. Methods Enzymol. 1980;64:192–226. doi: 10.1016/s0076-6879(80)64010-5. [DOI] [PubMed] [Google Scholar]
- Oen H., Wu C. W. DNA-dependent single-step addition reactions catalyzed by Escherichia coli RNA polymerase. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1778–1782. doi: 10.1073/pnas.75.4.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou C., Dorizzi M., Ninio J. A memory effect in DNA replication. Biochimie. 1984 Feb;66(2):115–119. doi: 10.1016/0300-9084(84)90199-8. [DOI] [PubMed] [Google Scholar]
- Papanicolaou C., Lecomte P., Ninio J. Mnemonic aspects of Escherichia coli DNA polymerase I. Interaction with one template influences the next interaction with another template. J Mol Biol. 1986 Jun 5;189(3):435–448. doi: 10.1016/0022-2836(86)90315-3. [DOI] [PubMed] [Google Scholar]
- Rappaport J., Weinmann R. Purine triphosphate beta-gamma bond hydrolysis requirements for RNA polymerase II transcription initiation and elongation. J Biol Chem. 1987 Dec 25;262(36):17510–17515. [PubMed] [Google Scholar]
- Shimamoto N., Wu C. W. Mechanism of ribonucleic acid chain initiation. 1. A non-steady-state study of ribonucleic acid synthesis without enzyme turnover. Biochemistry. 1980 Mar 4;19(5):842–848. doi: 10.1021/bi00546a003. [DOI] [PubMed] [Google Scholar]
- Shimamoto N., Wu C. W. Mechanism of ribonucleic acid chain initiation. 2. A real time analysis of initiation by the rapid kinetic technique. Biochemistry. 1980 Mar 4;19(5):849–856. doi: 10.1021/bi00546a004. [DOI] [PubMed] [Google Scholar]
- Sylvester J. E., Cashel M. Stable RNA-DNA-RNA polymerase complexes can accompany formation of a single phosphodiester bond. Biochemistry. 1980 Mar 18;19(6):1069–1074. doi: 10.1021/bi00547a004. [DOI] [PubMed] [Google Scholar]
- Vaisius A. C., Wieland T. Formation of a single phosphodiester bond by RNA polymerase B from calf thymus is not inhibited by alpha-amanitin. Biochemistry. 1982 Jun 22;21(13):3097–3101. doi: 10.1021/bi00256a010. [DOI] [PubMed] [Google Scholar]
- von Hippel P. H., Bear D. G., Morgan W. D., McSwiggen J. A. Protein-nucleic acid interactions in transcription: a molecular analysis. Annu Rev Biochem. 1984;53:389–446. doi: 10.1146/annurev.bi.53.070184.002133. [DOI] [PubMed] [Google Scholar]


