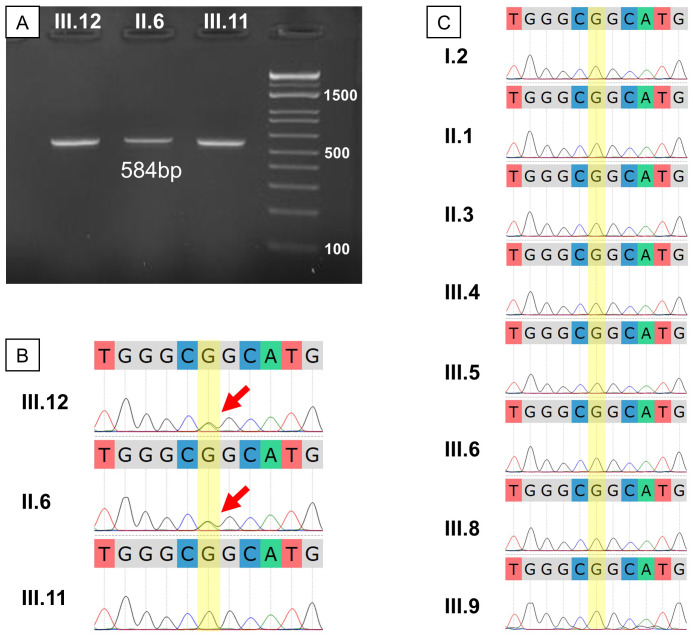
Figure 4.
Genetic result validation and extended testing for the studied family. (A) Gel electrophoresis of PCR products showed the expected fragment size of about 584 bp; (B) Sanger sequencing of the proband’s immediate family showed a heterozygous G>A mutation in III.12 and II.6; (C) Sanger sequencing for the extended family showed no other G>A mutation carrier among those who consented to the test.