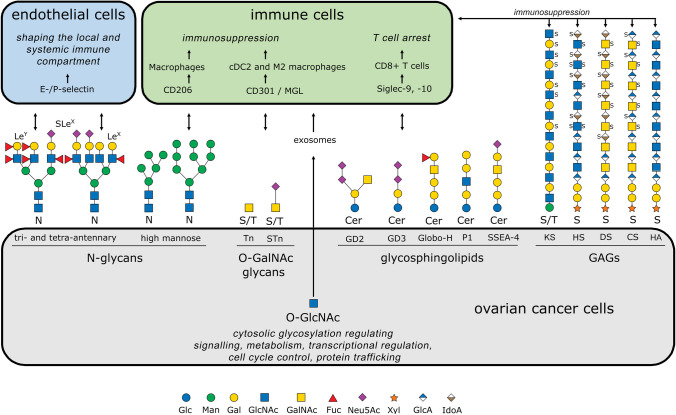
Fig. 1.
Schematic representation of aberrant glycan structures expressed in ovarian cancer cells (gray box) in modified IUPAC-condensed nomenclature via the web application GlycoGlyph [167]. The corresponding glycan-binding receptors on the endothelium (blue) and immune cells (green) are depicted, along with the cellular effects they mediate. Double-headed arrows indicate interactions between glycans and glycoreceptors. Terminal Lewis structures on N-glycans interact with E- and P-selectins on endothelial cells, playing a crucial role in shaping both local and systemic immune responses. High-mannose glycans are recognized by CD206 on macrophages and, like O-glycosidic Tn and STn antigens, contribute to an immunosuppressive environment through engagement of CD301 on CDC2 and M2 macrophages. In ovarian carcinoma, tumor-specific glycosphingolipids induce T-cell arrest by interacting with Siglec-9 and Siglec-10 on CD8+ T cells, further promoting immune evasion. Additionally, glycosaminoglycans (GAGs) expressed in ovarian carcinoma, such as keratan sulfate (KS-III), contribute to immunosuppression. The heterogeneous sulfation patterns of these GAGs serve as further examples of their complexity. Cytosolic proteins from ovarian carcinoma cells, modified by O-GlcNAc, can be transported to the tumor microenvironment via exosomes and subsequently internalized by immune cells. This mechanism supports immune evasion and further undermines the host immune response against the tumor. Fuc, fucose; Gal, galactose; GalNAc, N-acetylgalactosamine; Glc, glucose; GlcA, glucuronic acid; GlcNAc, N-acetylglucosamine; IdoA, iduronic acid; Man, mannose; Neu5Ac, N-acetylneuraminic acid (sialic acid); Xyl, xylose; S, sulfate group; KS, keratan sulfate; HS, heparan sulfate; DS, dermatan sulfate; CS, chondroitin sulfate; HA, hyaluronic acid; Cer, ceramide