Figure 1.
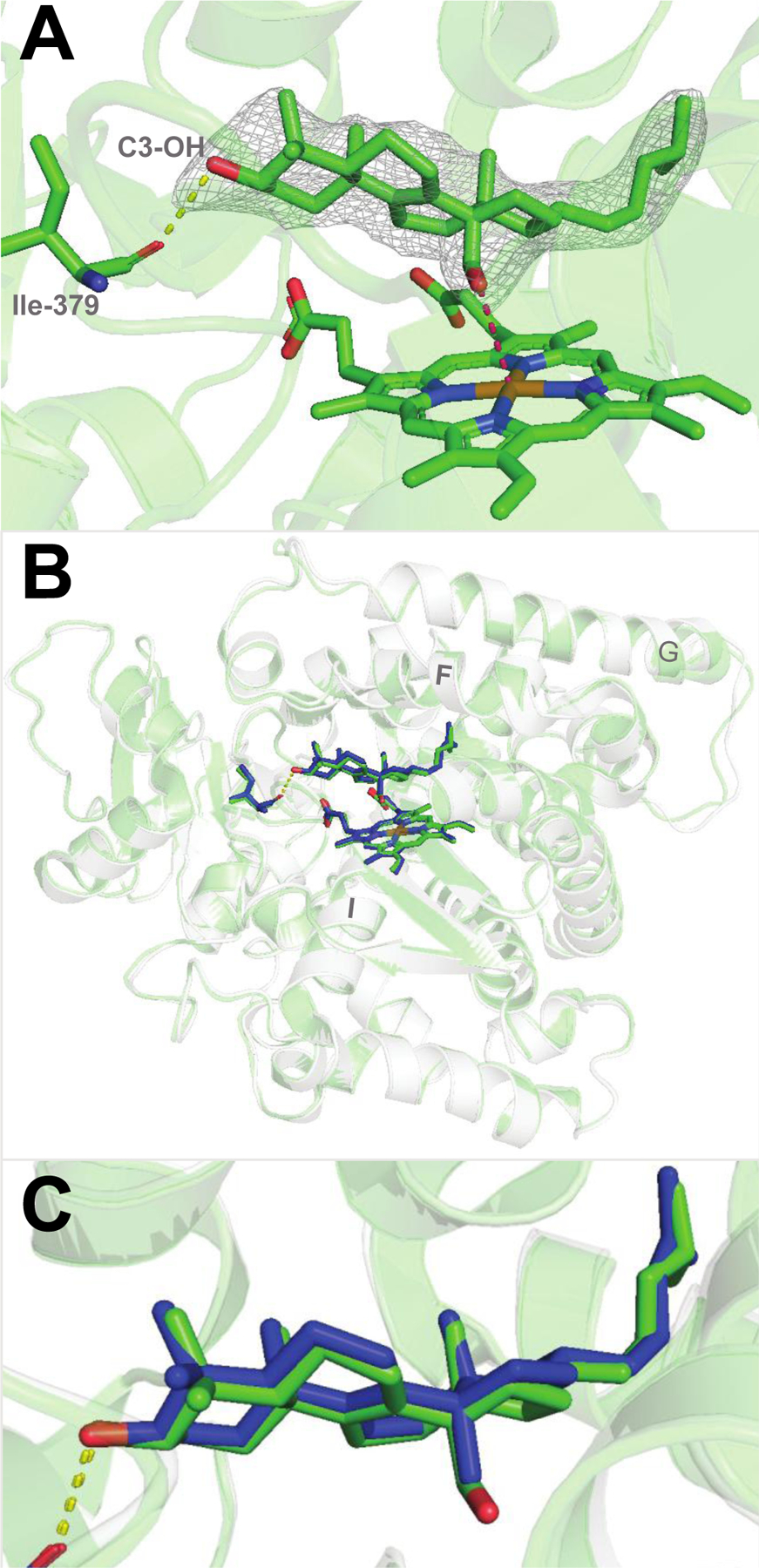
Substrate-bound human P450 51A1. (A) Binding mode of the 14α-aldehyde reaction intermediate inside the enzyme active site (PDB 8SS0, 2.25 Å.). The 2Fo-Fc electron density map within 1.6 Å of the sterol atoms is shown as gray mesh and contoured at 1.5σ. The H-bond between the C3-OH of the sterol molecule and the main chain oxygen of Ile-379 is depicted as yellow dashes. The distance between the aldehyde oxygen and the heme iron is 3.5Å (pink dashes). (B) Superimposition with the lanosterol-bound structure (PDB 6UEZ): the carbon atoms of lanosterol are colored in blue, the ribbon is transparent light-gray, and the rmsd of all Cα-atoms is 0.47 Å. (C) Enlarged view of the superimposed molecules of lanosterol and the 14α-aldehyde intermediate of 24,25-dihydrolanosterol.
