Figure 3.
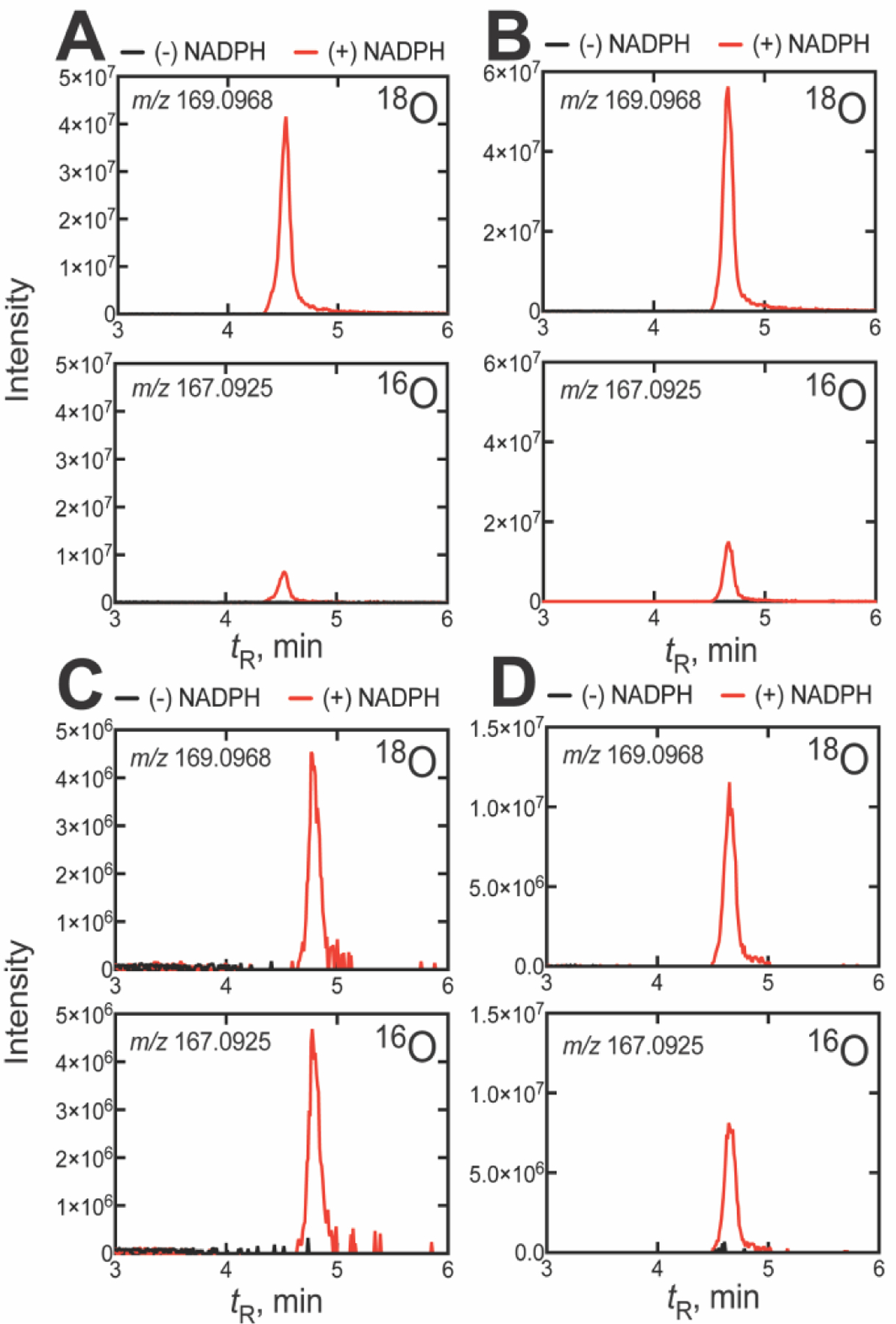
UPLC-HRMS analysis of formic acid formed from 14α-CDO dihydrolanosterol by other P450 51A enzymes in an atmosphere of 18O2. The two frames show the 18O (m/z 169.0968) and 16O (m/z 167.0925) channels for the formic acid derivative formed from the P450 51A enzymes of (A) N. fowleri, (B) C. albicans, (C) T. brucei, and (D) T. cruzi. All m/z values are calculated for the derivatized pyridyl formate esters. (The corresponding raw chromatographic traces for panel C are presented in Figure S4).
