Abstract
Objectives
To identify an optimal pediatric musculoskeletal ultrasound (MSUS) protocol for the detection of knee arthritis in patients with juvenile idiopathic arthritis (JIA) including a comparison with existing protocols. Secondary aims were to correlate MSUS-identified B-Mode (BM) and Power Doppler-Mode (PD) synovitis with clinical findings.
Methods
Consecutive JIA patients with confirmed knee arthritis after clinical examination underwent a thorough MSUS study protocol which included views identified and consented by the Pediatric Rheumatology european Society (PReS) Imaging Working Party for the detection of synovitis. In total eight views including measurement of the suprapatellar recess were included. Scoring of synovitis followed the pediatric OMERACT criteria (BM and PD severity grading 0 to 3). Interobserver reliability of BM and PD was tested before study begin. Previously published MSUS protocols for knee synovitis were also identified from the literature and their scan protocols compared to identify differences in sensitivity for synovitis according to the number and specific type of views included. Finally, a clinically applicable MSUS protocol for knee synovitis could be proposed.
Results
In 114 patients with clinically active knee inflammation, BM positivity (grading ≥ 1) was most frequently detected in the suprapatellar longitudinal and transverse scans performed in any positioning (frequency 97–99% in suprapatellar longitudinal in 30° or neutral respectively). PD positivity was however higher in these views performed in 30° flexion compared to neutral. Intrasynovial PD positivity (grading ≥ 1) was most frequently detected in the lateral parapatellar (69%, sensitivity 0.68, specificity 0.98), medial parapatellar (frequency 67%, sensitivity 0.67, specificity 1.0), the longitudinal lateral (68%, sensitivity 0.67, specificity 0.98) and suprapatellar transverse in 30° (frequency 64%, sensitivity 0.64, specificity 1.0). A combination of five views was the most sensitive for BM and PD synovitis. The suprapatellar recess size was analyzed by age and gender. For each group, the recess was wider in knees with arthritis than without (p < 0.001). Interobserver reliability of BM and PD positivity showed 85% agreement, with kappa 0.74 (very good). Three published studies with knee synovitis MSUS protocols were identified, which included a range of 1–3 views. Evaluation of the sensitivity of positive PD findings of each of these protocols reached a range of 53–83%; the highest sensitivity (91%) was achieved with the 5 views as identified by this study. These five views were therefore combined to form the Pediatric Internationally agreed Ultrasound (PIUS) knee protocol.
Conclusion
BM and PD positivity reliably correlated with the identification of pathological findings in knees of patients with JIA. From an internationally agreed protocol of eight images, a combination of five showed the greatest sensitivity for synovitis. This protocol, termed ‘PIUS-Knee’ performed well when compared to existing protocols.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12969-024-01029-4.
Keywords: Juvenile idiopathic arthritis, Knee synovitis, Ultrasound protocol, Intrasynovial hypervascularization
Key messages
• Synovitis detected using B-Mode and Doppler-Mode Musculoskeletal Ultrasound (MSUS) correlated strongly with clinical examination findings.
• A combination of five specific MSUS views achieved the most sensitive B-Mode and Doppler-Mode protocol for knee synovitis.
• This optimized knee examination ultrasound protocol termed PIUS-Knee (Pediatric Internationally Agreed Ultrasound) has high sensitivity for detecting intrasynovial hypervascularity.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12969-024-01029-4.
Introduction
Pediatric musculoskeletal ultrasound (MSUS) can play a large role in the interpretation of unclear clinical findings and has been tested in protocols both as an alternative and as an adjunct to clinical practice [1, 2]. However, multiple techniques and protocols exist [3–7]. Specific advantages of MSUS are the discrimination of tenosynovitis from arthritis, e.g. in the ankle region [8], the differentiation of active from residual findings or identification of differential diagnoses [9–14]. However, few studies describe the development of MSUS examination protocols used in juvenile idiopathic arthritis (JIA) and there remains a lack of internationally agreed protocols with standardized planes and positioning which affects the sensitivity for effusion or synovial hypervascularization [4, 15, 16]. Existing imaging protocols for the knee joint include a range of views based on the superior, medial and lateral knee compartments, performed in partial flexion or neutral position. Posterior and infrapatellar views are additionally required in routine knee MSUS examination to assess for popliteal cysts and infrapatellar pathologies as bursitis or enthesitis which are common in JIA. Therefore, the development of a sensitive but also practical protocol has been limited by the breadth of imaging positions and views possible.
Newer techniques, sensitive Doppler software and the inclusion of alternative planes as standard have encouraged international consensus groups to improve and define pediatric specific examination protocols [5, 7, 13, 17–23]. A semi-quantitative ultrasound score for the evaluation of B-Mode (BM) and conventional power Doppler (PD) published by the Outcome Measures in Rheumatology (OMERACT) Working Group has aided the evaluation of imaging protocols [24].
The PReS imaging working party initiated this study to develop the most sensitive knee joint specific MSUS protocol with feasible clinical use to advance an internationally unified approach. Therefore, the specific aims of this study were to identify an optimal pediatric MSUS protocol for the detection of knee synovitis in patients with JIA by evaluating validated semi-quantitative MSUS measures of synovitis using BM and PD in multiple scan views. Secondly, BM and PD measures of synovitis were evaluated with the aim of determining the most sensitive views to achieve a short but high sensitivity protocol for synovitis.
Methods
Patients aged ≤ 18 with JIA diagnosed according to the ILAR criteria and with clinically diagnosed active inflammation of at least one knee joint were eligible for the study. By eligible patients, both knees were scanned, including those without clinical arthritis as a control group for comparison. Clinical arthritis of the knee was defined as the presence of a palpable joint effusion and limited range of motion (LOM) or pain on clinical examination. All patients received a standard complete clinical examination of all large peripheral joints and a standard physical examination to exclude co-existing diagnoses e.g. infection. C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were determined as routine laboratory investigations. Disease activity was recorded using the validated JADAS-10 score.
Patients who fulfilled the inclusion criteria and had confirmed arthritis of at least one knee with arthritis after clinical examination, were eligible for recruitment. Exclusion criteria included active infection, presence of other autoimmune diseases, injuries, other musculoskeletal disorders and weight above 90 percentile. Written patient (when aged > 6 years) and parental consent was obtained before study inclusion. Patients were consecutively recruited between 2019 and 2023 in each participating center (total nine) until the required ‘n’ (102 knees) for statistical analysis was reached. Ethical approval was obtained from the ethic commissions of the University of Giessen and the Ärztekammer Westfalen-Lippe, Germany. MSUS examiners were pediatric rheumatology specialists (≥ 5 years’ experience in MSUS) and also members of the imaging groups of the PReS (Pediatric Rheumatology european Society) and/or the GKJR (German Pediatric Rheumatology Society). MSUS examiners performed the scans and grading of BM and PD.
Ultrasound assessment
All participants underwent MSUS examination of both knees following the study protocol which was internationally agreed and tested in an investigator face-to-face meeting in January 2019 in Munich, which included the practical application of the study protocol. Eight different standard views incorporating different positions were included as follows:
The longitudinal suprapatellar scan (in neutral position with muscle tension and in 30° flexion).
The transverse suprapatellar scan (in neutral position with muscle tension and in 30° flexion).
Both transverse parapatellar scans (lateral and medial).
Both longitudinal scans (lateral and medial, encompassing the meniscus region).
The suprapatellar recess (in longitudinal and transverse, in 30° and neutral position) was also measured in each examination. The examination of all views was performed with a linear transducer (minimal BM frequency 12 MHZ) and the most sensitive setting for PD in each center, adapted to minimize artifacts. Investigators used their own ultrasound devices as per routine clinical use.
Analysis of ultrasound findings
For each scan in the protocol, the highest possible BM and PD finding was documented. BM findings indicate effusion and synovial hypertrophy, and are quantified by the internationally agreed synovitis score of the Pediatric OMERACT group (grade 0 = no effusion, grade I = mild effusion and/or synovial hypertrophy, grade II = effusion and/or synovial hypertrophy leading to convex shaped recess, grade III = large effusion and/or synovial hypertrophy). BM positivity refers to any BM grading of ≥ 1. PD findings were also graded by the internationally consented synovitis score by the Pediatric OMERACT group (grade 0 = no intrasynovial Doppler signal, grade 1 = few individual dots of synovial Doppler signals, grade 2 = confluent Doppler signals, but representing less than 30% of the visible synovial tissue and grade 3 = confluent Doppler signals in more than 30% of the visible synovial tissue) [25].
The recess size (maximal height in mm) measured in the suprapatellar longitudinal view (maximal anterior-posterior diameter) and transverse view (maximal anterior-posterior diameter perpendicular to the femur) were recorded for all examinations.
An interobserver US reliability test for the BM and PD positivity was performed using 20 still images with different degrees of synovitis with BM and PD grades of 0–3 evaluated by ten MSUS examiners.
Finally, the results of the MSUS BM and PD findings in the JIA group were compared to the results of those in the healthy knee control group. Clinical examination was taken as the gold standard in the determination of arthritis. The most sensitive and practical combination of views for BM and PD were collated into the PReS Imaging Working group agreed “PIUS” protocol which was compared numerically to existing protocols for the MSUS evaluation of knee arthritis by application of their protocol for detecting intrasynovial hypervascularization within our patient group and the resulting sensitivity.
Statistical analysis
Descriptive statistics were used to summarize the clinical, laboratory and sonography data collected as follows: frequency and percentage for categorical variables; mean, median, standard deviation, minimum and maximum values for continuously distributed variables. The Chi-square test or Fischer’s exact test and Student’s t-test or Mann-Whitney U test were used to compare the proportions and variables between groups. A p value of < 0.05 was considered as statistically significant.
For the evaluation of diagnostic accuracy for categorical variables, the sensitivity, specificity, predictive values, likelihood ratios, accuracy and diagnostic odds ratio were evaluated with a 95% confidence interval for BM and PD scores for each view plane. For the statistical analysis of BM and PD as 2-variable categorical variables, the effusion and synovitis grading of ≥ 1 was defined as positive and 0 negative. A receiver-operating characteristic (ROC) curve was used to determine the cut-off values with the best sensitivity and specificity for the MSUS detection of arthritis. The predictive ability of MSUS was also evaluated using likelihood ratios. For the area under the ROC curve (AUC), discrimination ability was evaluated according to the following categories: 0.90-1 = excellent, 0.80–0.90 = good, 0.70–0.80 = moderate, 0.60–0.70 = poor, and 0.50–0.60 = unsuccessful. The optimal cutoff points for predicting pathologic effusion were based on the highest Youden index.
Reliability for the interobserver analysis agreement BM and PD positivity was determined using Fleiss Kappa. A kappa score of < 0.2 is considered poor, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 good and 0.81-1.00 excellent. All statistical analyses were performed using the IBM SPSS software version 25 and MedicReS Good Biostatistical Consultancy Standards (www.e-picos.com, NY, New York software and MedCalc statistics).
Results
Patients
114 patients (80 female, 70%) aged 11.0 (mean, SD ± 4.3) years with JIA diagnosed according to the ILAR criteria and current active inflammation of at least one knee joint as determined by clinical examination (‘clinical arthritis’) were included. 92 patients (81%) had oligoarticular arthritis, 18 (16%) had polyarticular arthritis. The JADAS-10 score median (min-max) was 11.3 (3.0–30.0) for all JIA patients. The demographic, disease and clinical characteristics of the patients are shown in Table 1. Clinical arthritis was present in 137 knees in 114 patients; 23/114 patients had bilateral knee arthritis. 66 clinically healthy knees from the 114 patients formed the control group and were subjected to the same study ultrasound protocol as knees with clinical arthritis. Patients with unilateral arthritis had statistically significant lower JADAS10 scores (mean 10.5 ± 3.6 SD vs. 12.6 ± 5.4) and lower number of total active joints (1.5 ± 1.4 SD vs. 2.9 ± 4.6) compared to the whole patient cohort, but did not otherwise differ. MSUS examinations were performed by 11 different examiners and 72/137 (52%) knee ultrasound examinations were performed by an examiner blinded to the clinical findings.
Table 1.
Demographic and clinical characteristics of patients with knee arthritis
| Patients with knee arthritis | n = 114 (%) |
|---|---|
| Gender | |
| Female | 80 (70.2) |
| Male | 34 (29.8) |
| Mean age ± SD/ median (min-max) | 11.0 ± 4.3 / 11.6 (1–18) |
| Category of JIA | |
| Oligoarticular JIA | 92 (80.7) |
| Polyarticular JIA | 18 (15.8) |
| Enthesitis related arthritis | 2 (1.8) |
| Psoriatic arthritis | 2 (1.8) |
| Previous Intraarticular steroid application of the knee joint(-s) | 72/114 (63.1) |
| Unilateral | 42/114 (36.8) |
| Bilateral | 30/114 (26.3) |
| ANA positivity | 97/112 (86.6) |
| HLAB-27 positivity | 11/94 (11.7) |
| RF positivity | 3/109 (2.8) |
| Anti-CCP positivity | 3/63 (4.8) |
| Clinical signs of arthritis | |
| Palpable joint effusion | 107/110 (97.3) |
| LOM | 75/102 (73.5) |
| Pain | 83/111 (74.8) |
| Swelling | 108/112 (96.4) |
| Current medication use | 81/113 (71.7) |
| Biologicals | 6/113 (5.3) |
| Methotrexate | 36/113 (32) |
| Systemic steroid | 6/113 (5.3) |
| Other medications | 61/113 (54) |
| Current eye involvement | 13/113 (11.5) |
| Mean ± SD / median (min-max) | |
| Parameters of disease activity | |
| JADAS10 score | 12.6 ± 5.4 / 11.3 (3.0–30.0) |
| Number of active joints | 2.9 ± 4.6 / 2.0 (1–45.0) |
| VAS activity of patient | 4.6 ± 2.2 / 5.0 (0–9.0) |
| VAS activity of physician | 4.9 ± 1.9 / 3 (1.0–10.0) |
| Erythrocyte sedimentation ratio (mm/h) | 19.0 ± 18.7 / 11.5 (1.0–84.0) |
| C-reactive Protein (mg/dL) | 1.3 ± 2.7 / 0.3 (0-23.7) |
Abbreviations: ANA anti-nuclear antibody, CCP Anti-cyclic citrullinated peptide, JIA juvenile idiopathic arthritis, LOM limted range of motion, min-max minimum-maximum, RF rheumatoid factor, SD standard deviation, VAS visual analogue scale
Ultrasound findings: effusion and synovial hypertrophy
MSUS identified effusion and/or synovial hypertrophy (BM positivity) in at least one of the imaging views in all patients with clinical arthritis. Comparing single images, BM positivity was most frequently detected in the suprapatellar scans, with at least 95% of knees showing positivity depending on the specific view (Table 2). The level of positivity of the BM score of each individual image varied within the protocol (Supplement Table 1), with the highest BM grade (score 3) also being most often found in one of the four suprapatellar images (longitudinal or transverse in flexion or neutral). Low grade (maximum grade 1) BM positivity was also detected in 8/66 control knees in at least one of the suprapatellar views. In 5 of these 8 cases, all other views had a BM grade of 0. In the other 3 cases, 2 cases had BM Grade 1 positivity also in the lateral and medial parapatellar views and control knee additionally had BM Grade 1 positivity in the medial and lateral longitudinal views.
Table 2.
Positivity of B-Mode and Power-Doppler-Mode in Arthritis and Control Groups
| Arthritis group (n = 137 knee joints) | Control group (n = 66 knee joints) | |||||||
|---|---|---|---|---|---|---|---|---|
| B-Mode findings | ||||||||
| Region |
Positive n (%) |
Negative n (%) |
Positive n (%) |
Negative n (%) |
P* | |||
| 30° | ||||||||
| Longitudinal | 133 (97.1) | 4 (2.9) | 4 (6.1) | 62 (93.9) | < 0.001 | |||
| Transverse | 131 (95.6) | 6 (4.4) | 4 (6.1) | 62 (93.9) | < 0.001 | |||
| 0° | ||||||||
| Longitudinal | 135 (98.5) | 2 (1.5) | 2 (3.0) | 64 (97.0) | < 0.001 | |||
| Transverse | 134 (97.8) | 3 (2.2) | 3 (4.5) | 63 (95.5) | < 0.001 | |||
| Long. Lateral | 118 (86.1) | 19 (13.9) | 2 (3.0) | 64 (97.0) | < 0.001 | |||
| Long. Medial | 106 (77.4) | 31 (22.6) | 1 (1.5) | 65 (98.5) | < 0.001 | |||
| PP-lateral | 122 (89.1) | 15 (10.9) | 2 (3.0) | 64 (97.0) | < 0.001 | |||
| PP-medial | 121 (88.3) | 16 (11.7) | 2 (3.0) | 64 (97.0) | < 0.001 | |||
| Power-Doppler Mode findings | ||||||||
| 30° | ||||||||
| Longitudinal | 72 (52.6) | 65 (47.4) | 0 (0) | 66 (100) | < 0.001 | |||
| Transverse | 88 (64.2) | 49 (35.8) | 0 (0) | 66 (100) | < 0.001 | |||
| 0° | ||||||||
| Longitudinal | 59 (43.4) | 77 (56.6) | 0 (0) | 66 (100) | < 0.001 | |||
| Transverse | 75 (54.7) | 62 (45.3) | 0 (0) | 66 (100) | < 0.001 | |||
| Long. Lateral | 93 (67.9) | 44 (32.1) | 1 (1.5) | 65 (98.5) | < 0.001 | |||
| Long. Medial | 69 (50.4) | 68 (49.6) | 0 (0) | 66 (100) | < 0.001 | |||
| PP-lateral | 94 (68.6) | 43 (31.4) | 1 (1.5) | 65 (98.5) | < 0.001 | |||
| PP-medial | 92 (67.2) | 45 (32.8) | 0 (0) | 66 (100) | < 0.001 | |||
Abbreviations: Long Longitundinal
*Fisher’s Exact Test
The sensitivity and specificity with 95% confidence intervals for each individual view for the detection of synovitis in knees with and without clinical synovitis are shown in Supplement Table 2, for both the evaluation of BM and PD. The sensitivity of the suprapatellar scans combined was 100%, specificity 87.8%, positive predictive value (PPV) 94.4% and the negative predictive value (NPV) 100%.
The positioning of the patient in 30° flexion or neutral for the suprapatellar longitudinal or transverse views was not associated with a statistically significant difference in the BM positivity. However PD positivity was more frequent and more sensitive in 30° flexion in the transverse scan compared to in neutral.
Ultrasound findings: intrasynovial hypervascularisation
Intrasynovial hypervascularization (PD positivity) could be identified in at least one view of the scan protocol in 124/137 knees (102/114 patients) with clinical arthritis (Table 2). The sensitivity (level of positivity of PD score) of each individual imaging view within the protocol varied, as with the evaluation of BM (Supplement Table 1). The highest grade of PD positivity (grade 3) was most frequently found in the lateral parapatellar (n = 20/137, 15%) and lateral longitudinal scans (n = 15/137, 11%). An example of synovitis in the lateral longitudinal image in a knee with clinical and MSUS arthritis, with BM and PD positivity is shown in Fig. 1. Additionally, the probe positioning and corresponding MRI image is shown.
Fig. 1.
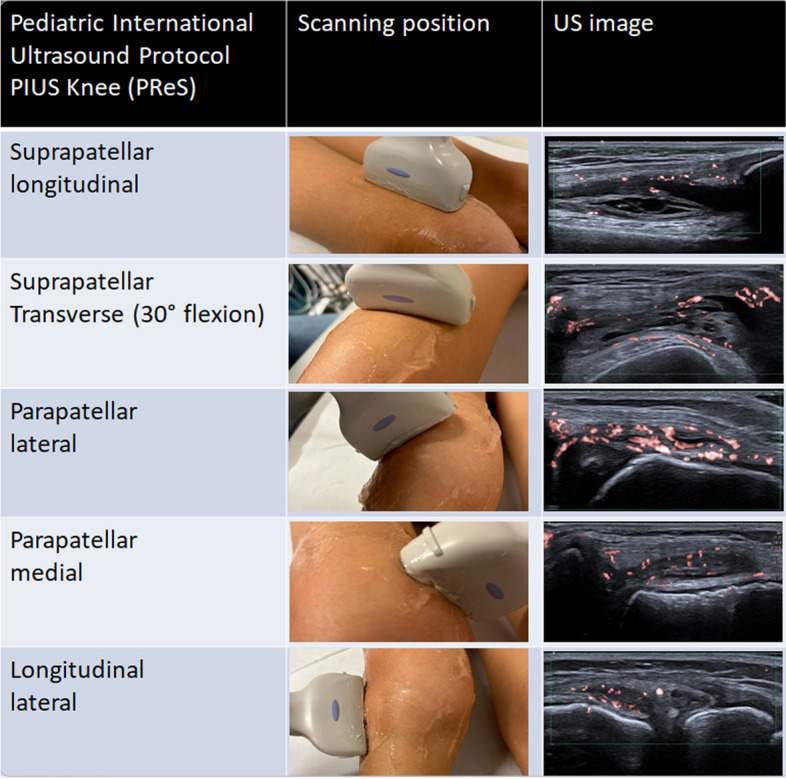
The new PReS „PIUS protocol“ for the sensitive detection of intrasynovial hypervascularization in the pediatric knee joint includes five views for the optimal detection of arthritis, as shown
PD positivity of any grade was most frequently found in the medial (n = 92/137, 67% of knees) and lateral parapatellar scans (n = 94/137, 69%), the longitudinal lateral (n = 93/137, 68%) and the transverse suprapatellar scan in 30° flexion (88/137, 64%) in knees with clinical arthritis (Table 2). As these four views indicated the highest likelihood of identifying PD positivity, we evaluated the potential for these four imaging views to be used as screening planes for the examination protocol. PD positivity in at least one of the four views had a sensitivity of 98.4%, specificity of 89%, positive predictive value (PPV) of 99.1% and negative predictive value (NPV) of 81.2% for the identification of knee with clinical arthritis. Low grade PD positivity (PD = 1) was identified in one clinically healthy control knee at the longitudinal lateral and parapatellar longitudinal images.
Ultrasound findings: suprapatellar recess size
Recess size was analyzed in subgroups according to the age and gender of patients, to achieve age and sex-matched healthy controls. The recess size was statistically significantly larger in knees with clinical arthritis compared to controls in both the suprapatellar longitudinal and transverse scans regardless of the age and gender (Table 3). ROC analysis was used to determine cut-offs for recess size in both these scan views. The cut-off of 3.5 mm and 3.0 mm for the suprapatellar longitudinal and transverse views respectively for the identification of arthritis resulted in an area under the curve of at least 95%, regardless of whether performed in 0 or 30° flexion. The sensitivity was at least 86% and specificity at least 95%, depending on whether the scan was performed in neutral or 30° flexion and if the transverse or longitudinal view suprapatellar was performed (Supplement Table 3).
Table 3.
Comparison of the suprapatellar recess size as measured by ultrasound in patients with and without clinical arthritis
| Suprapatellar Recess size Mean ± SD Median (Min-Max) | |||||
|---|---|---|---|---|---|
| Knees with clinical arthritis | Knees without clinical arthritis | P Value | |||
|
1–3 years Boys (n = 0) Girls (n = 11) |
n/a 9.9 (6.7–10.7) 3.9–12.0 |
1–3 years Boys (n = 0) Girls (n = 3) |
n/a 1.3 (0.7-§) 0.7–1.5 |
< 0.001 | |
|
4–6 years Boys (n = 4) Girls (n = 14) |
5.8 (2.5–7.1) 1.5–7.6 7.9 (4.6–9.3) 3.6–10.2 |
4–6 years Boys (n = 1) Girls (n = 7) |
-§ 1.0 (0.7–1.4) 0.1–2.2 |
0.004 | |
|
7–9 years Boys (n = 10) Girls (n = 12) |
6.2 (3.5–10.2) 2.1–12.0 9.3 (8.6–11.5) 7.0-13.4 |
7–9 years Boys (n = 5) Girls (n = 2) |
1.3 (1.0-1.5) 1.0-1.8 1.0 (0.9-§) 0.9–1.2 |
< 0.001 | |
|
10–12 years Boys (n = 7) Girls (n = 28) |
7.6 (4.9–23.6) 4.3–69.0 7.8 (5.7–9.5) 1.9–13.9 |
10–12 years Boys (n = 3) Girls (n = 20) |
1.9 (1.2-§) 1.2–4.3 1.5 (1.0-1.9) 0.6–2.6 |
< 0.001 | |
|
13–15 years Boys (n = 12) Girls (n = 21) |
11.2 (9.2–16) 4.0-18.3 7.2 (5.6–8.9) 3.6–13.1 |
13–15 years Boys (n = 5) Girls (n = 10) |
1.2 (0.5–2.4) 0-3.4 1.7 (1.1–2.5) 0-3.4 |
< 0.001 | |
|
16–18 years Boys (n = 8) Girls (n = 10) |
9.7 (7.3–10.7) 5.6–16.8 6.6 (4.7–11.6) 3.7–17.5 |
16–18 years Boys (n = 6) Girls (n = 4) |
0.8 (0-1.6) 0-1.8 1.1 (0.8–1.3) 0.7–1.4 |
< 0.001 | |
min-max minimum-maximum, SD standard deviation
§incalculable
Evaluation of a combined knee ultrasound protocol
Five of the eight images could be combined to establish a short but clinically sensitive protocol for the identification of arthritis. This combination included the suprapatellar longitudinal scan and the transverse suprapatellar scan in 30° flexion, the medial and lateral parapatellar scans and the lateral longitudinal scan (Fig. 2). These five images extracted from the study protocol together achieved a combined sensitivity of 91% for the identification of Doppler synovitis.
Fig. 2.
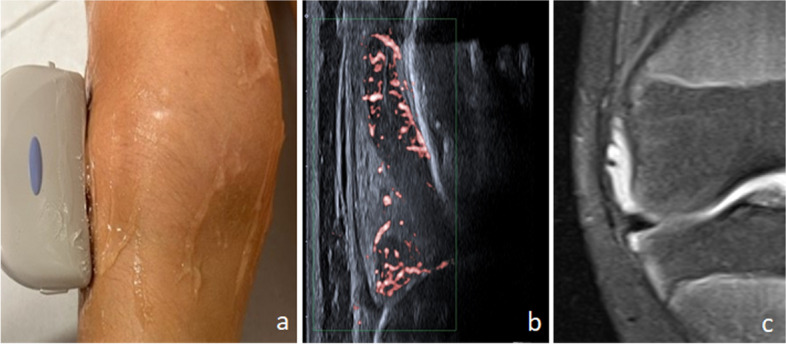
Images illustrate the longitudinal lateral view scanning position (a) and corresponding ultrasound image (b) in comparison to the MRI image of the corresponding plane (c)
Evaluation of the external protocols identified is summarized in Table 4. The application of the identified alternative scan protocols to this dataset revealed a sensitivity for the identification of hypervascularization ranging from 53 to 85%. Higher sensitivity was achieved with protocols which included more views. Therefore, the five-image protocol established for synovitis in this study achieved a higher sensitivity for the detection of synovitis compared to the alternative protocols reviewed.
Table 4.
Comparison of the sensitivity of the PReS study ultrasound protocol and different ultrasound protocols in our arthritis group
| Combinations | Combination I (6) | Combination II (5) | Combination III (7) |
Combination IV (unpublished PReS data) |
Combination V (PIUS protocol as suggested in this study) |
|---|---|---|---|---|---|
| Regions | Suprapatellar scan (longitudinal) | Suprapatellar scan (longitudinal 30°) |
Suprapatellar scan (longitudinal 30°) |
Suprapatellar scan (longitudinal 30°) | Suprapatellar scan (longitudinal 30°) |
| ------ | Medial parapatellar scan | ------ | Medial parapatellar scan | Medial parapatellar scan | |
| ------ | Lateral parapatellar scan | Lateral parapatellar scan | Lateral parapatellar scan | Lateral parapatellar scan | |
| ------ | ----- | ------ | Lateral longitudinal scan | Lateral longitudinal scan | |
| ------ | ------ | ------ | ------ | Suprapatellar scan (transversal 30°) | |
| B-Mode plus PD-Mode positivity | 52.6% | 82.5% | 76.6% | 85.4% | 90.5% |
Interobserver test
The interobserver-test for the evaluation of BM and PD positivity showed substantial agreement (kappa 0.848, p < 0.001, CI 95% 0.765–0.930) between the investigators.
Discussion
The knee is the most commonly affected site in JIA and has therefore been a focus for the investigation of MSUS studies of normative and pathological findings over the last 15 years [4, 26–30]. Increasing standardization, technical developments and newer Doppler techniques have encouraged the optimization of examination protocols to reliably diagnose knee arthritis [17, 31]. A large German study by the GKJR reported age related normal findings of pediatric knee joints using MSUS in 2016 which formed the basis of characterizing pathological findings [29]. More recently, studies have reported significant differences between normal and pathological findings in MSUS, although MSUS results which conflict with clinical findings are also reported [2, 5, 9, 32, 33].
Individual views were more likely to show BM than PD positivity. However, BM alone is insufficient for the determination of the presence of synovitis. Contralateral knees without clinical synovitis were used as a control group as they were easily available, and clinically significantly differed from the knees with clinical arthritis. Subclinical synovitis could still potentially be present and potentially detectable by ultrasound. However, it was not the aim of this study to evaluate the sensitivity of the MSUS protocol for the detection of relevant subclinical synovitis, though this could be a focus of future studies. In this study, eight control knee joints which had no LOM, palpable joint effusion or pain present had BM positivity. The aforementioned GKJR study indicated healthy children can have low grade but positive BM findings and larger suprapatellar recess size without co-existing pathology [29]. However, no intrasynovial hypervascularization is expected in healthy children [27, 34, 35].
The suprapatellar recess size could distinguish non-arthritic and pathological knee joint effusions well in this study (sensitivity > 86%, specificity > 95% depending on positioning) and a cut-off of 3.5 mm (longitudinal suprapatellar) or 3.0 mm (transverse suprapatellar) had a high AUC for determining a pathological joint effusion. However, our patients were pre-selected for having a JIA diagnosis, and recess width remains unspecific as it is affected in multiple pathologies including traumatic or hemorrhagic causes.
The inclusion of multiple sensitive views for BM and evaluation of intrasynovial hypervascularization using PD are therefore important additional criteria for the specific detection of pathology. However, whilst PD increasingly influences the interpretation of inflammation in joints [4, 27, 34, 35], it’s use and sensitivity in protocols has been so far variable and with low sensitivity [5–7]. In this study, intrasynovial hypervascularization was most frequently found in the medial parapatellar, lateral parapatellar, longitudinal lateral and the transverse suprapatellar scan in 30° flexion. These views capture more superficial lying synovial vessels in comparison to those involving the longitudinal suprapatellar plane, allowing easier detection by the ultrasound probe. Evaluation of PD in these views therefore aided a more specific discrimination of active inflammation from non-arthritic findings.
Intrasynovial PD positivity was found only in one knee joint without clinical arthritis. Reviewing the finding, this image showed a small feeding vessel, rather than active inflammation. Published OMERACT data which reports vascularization in healthy knees has determined feeding vessels should not considered as a sign of inflammation. In contrast, 13 knees with BM positivity and clinical arthritis had no detectable PD positivity. These findings could represent a subset of patients with secondary clinical findings and a residual effusion rather than active inflammation.
This was the first study comparing the neutral position and 30 degree flexion position in pediatric MSUS suprapatellar scan examination. The sensitivity was slightly but not significantly higher with the neutral position for the suprapatellar longitudinal scan for BM positivity, but both positions showed a high specificity. However, PD positivity was more sensitive in the 30° flexion position when performing the transverse suprapatellar view. Whilst these results are encouraging for the identification of arthritis, the suprapatellar scan together with the recess height alone is insufficient to reliably discriminate active arthritis from non-arthritic findings.
Whilst MSUS protocols for examining standard regions in patients with JIA exist, specific protocols for individual joints are limited, though the knee has been the most intensively researched. Often protocols have focused on shorter protocols for ease of use in the clinical setting [6, 7]. However, this carries the risk of missing intrasynovial hypervascularization in some patients. Both these published protocols incorporated the longitudinal suprapatellar scans, whilst Sande et al. additionally included the lateral parapatellar scan for synovitis. When the alternative protocols were used on this data set, it resulted in a minimum sensitivity of only 53% and maximum sensitivity of 83% [5]. Using the five-image protocol as defined here, a sensitivity of 91% for BM and PD positivity in knees with clinical synovitis was reached with only an average 5–7 min scan time required for both knees.
Conclusion
In summary, our study was able to show that a protocol of five-images can safely identify synovitis and discriminate between clinically defined healthy and arthritis knees in patients with JIA. The five-image protocol incorporating BM and PD measures, termed the Pediatric International knee US or ‘PIUS’ Protocol, performed well compared to existing protocols and remains short enough for routine use in the clinic. An optimized MSUS knee protocol that can reliably detect inflammation is fundamental to allow dependable use in the treat-to-target approach, for example, for longitudinal monitoring of disease activity including the identification of subclinical inflammation or for the quantification of improvement after therapeutic interventions. Whilst the ability of MSUS to achieve these aims is not yet proven, studies are ongoing to address such questions [36].
Supplementary Information
Acknowledgements
We thank Heike Stapel for her support with study organsiation, data collation and analysis.
Abbreviations
- AUC
Area under the curve
- BM
B-Mode
- CRP
C-reactive protein
- ESR
erythrocyte sedimentation rate
- GKJR
German Pediatric Rheumatology Society
- JADAS-10
juvenile arthritis disease activity score, 10-point
- JIA
juvenile idiopathic arthritis
- LOM
limited range of motion
- MSUS
musculoskeletal ultrasound
- NPV
negative predictive value
- OMERACT
Outcome Measures in Rheumatology
- PD
Power Doppler-Mode
- PIUS-knee
Pediatric International agreed Ultrasound Knee synovitis protocol
- PPV
positive predictive value
- PReS
Pediatric Rheumatology european Society
- ROC
receiver-operating characteristic
- SD
Standard deviation
Authors’ contributions
DW, RT, MKL, LF, RB, MH and SSM conceived and planned the study. All authors recruited patients and performed ultrasound examinations. DW, HAD, FG, RT, MKL, RB, MH analyzed and interpreted the patient data. DW, FG, HAD, SV, SS, LF, FD, BS and SMM were major contributors in writing the manuscript. All authors read and approved the final manuscript.
Funding
This study recived partial funding support from Novartis. The funding body played no role in the design of the study or in the collection, analysis and interpretation of data or in writing the manuscript.
Data availability
This manuscript has additional data available as a supplement file.
Declarations
Ethics approval and consent to participate
Ethical approval was obtained from the ethic commissions of the University of Giessen and the Ärztekammer Westfalen-Lippe, Germany. Written patient (when aged > 6 years) and parental consent was obtained before study inclusion.
Consent for publication
Consent for publication of images was obtained from patients and their legal guardians.
Competing interests
The corresponding and last author is an associate editor for the Journal Pediatric Rheumatology.
Footnotes
The original online version of this article was revised: Following publication of the original article [1], we have been notified that the first and last author names had been incorrectly switched in production.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
10/29/2024
A Correction to this paper has been published: 10.1186/s12969-024-01032-9
References
- 1.Breton S, Jousse-Joulin S, Cangemi C, de Parscau L, Colin D, Bressolette L, et al. Comparison of clinical and ultrasonographic evaluations for peripheral synovitis in juvenile idiopathic arthritis. Semin Arthritis Rheum. 2011;41(2):272–8 Available from: http://www.sciencedirect.com/science/article/pii/S0049017211000023 . [DOI] [PubMed] [Google Scholar]
- 2.Collado P, Naredo E, Calvo C, Gamir ML, Calvo I, García ML, et al. Reduced joint assessment vs comprehensive assessment for ultrasound detection of synovitis in juvenile idiopathic arthritis. Rheumatol (United Kingdom). 2013;52(8):1477–84. [DOI] [PubMed] [Google Scholar]
- 3.Chauvin NA, Khwaja A. Imaging of inflammatory arthritis in children: Status and perspectives on the use of ultrasound, radiographs, and magnetic resonance imaging. Rheum Dis Clin North Am. 2016;42(4):587–606. [DOI] [PubMed] [Google Scholar]
- 4.Collado P, Vojinovic J, Nieto JC, Windschall D, Magni-Manzoni S, Bruyn GAW, et al. Toward standardized Musculoskeletal Ultrasound in Pediatric Rheumatology: normal age-related Ultrasound findings. Arthritis Care Res. 2016;68(3):348–56. [DOI] [PubMed] [Google Scholar]
- 5.Ting TV, Vega-Fernandez P, Oberle EJ, De Ranieri D, Bukulmez H, Lin C, et al. Novel ultrasound image acquisition protocol and scoring system for the pediatric knee. Arthritis Care Res (Hoboken). 2019;71(7):977–85. 10.1002/acr.23746. [DOI] [PubMed] [Google Scholar]
- 6.Shanmugavel C, Sodhi KS, Sandhu MS, Sidhu R, Singh S, Katariya S, et al. Role of power doppler sonography in evaluation of therapeutic response of the knee in juvenile rheumatoid arthritis. Rheumatol Int. 2008;28(6):573–8. 10.1007/s00296-007-0482-7. [DOI] [PubMed] [Google Scholar]
- 7.Sande NK, Bøyesen P, Aga A-B, Hammer HB, Flatø B, Roth J, et al. Development and reliability of a novel ultrasonographic joint-specific scoring system for synovitis with reference atlas for patients with juvenile idiopathic arthritis. RMD Open. 2021;7(2):e001581 Available from: http://rmdopen.bmj.com/content/7/2/e001581.abstract . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rooney ME, McAllister C, Burns JFT. Ankle disease in juvenile idiopathic arthritis: ultrasound findings in clinically swollen ankles. J Rheumatol. 2009;36(8):1725 LP – 1729 Available from: http://www.jrheum.org/content/36/8/1725.abstract . [DOI] [PubMed] [Google Scholar]
- 9.Magni-Manzoni S, Scirè CA, Ravelli A, Klersy C, Rossi S, Muratore V, et al. Ultrasound-detected synovial abnormalities are frequent in clinically inactive juvenile idiopathic arthritis, but do not predict a flare of synovitis. Ann Rheum Dis. 2013;72(2):223 LP – 228 Available from: http://ard.bmj.com/content/72/2/223.abstract . [DOI] [PubMed] [Google Scholar]
- 10.Rebollo-Polo M, Koujok K, Weisser C, Jurencak R, Bruns A, Roth J. Ultrasound findings on patients with juvenile idiopathic arthritis in clinical remission. Arthritis Care Res. 2011;63(7):1013–9. [DOI] [PubMed] [Google Scholar]
- 11.Lanni S, van Dijkhuizen EHP, Vanoni F, Viola S, Magnaguagno F, Magnano GM, et al. Ultrasound changes in synovial abnormalities induced by treatment in juvenile idiopathic arthritis. Clin Exp Rheumatol. 2018;36(2):329–34 Available from: http://europepmc.org/abstract/MED/29185965 . [PubMed] [Google Scholar]
- 12.De Lucia O, Ravagnani V, Pregnolato F, Hila A, Pontikaki I, Gattinara M, et al. Baseline ultrasound examination as possible predictor of relapse in patients affected by juvenile idiopathic arthritis (JIA). Ann Rheum Dis. 2018;77(10):1426 LP – 1431 Available from: http://ard.bmj.com/content/77/10/1426.abstract . [DOI] [PubMed] [Google Scholar]
- 13.Malattia C, Rinaldi M, Martini A. The role of imaging in juvenile idiopathic arthritis. Clin Immunol. 2018;14(8):681–94. 10.1080/1744666X.2018.1496019. [DOI] [PubMed] [Google Scholar]
- 14.Johnson K. Imaging of juvenile idiopathic arthritis. Pediatr Radiol. 2006;36(8):743–58. [DOI] [PubMed] [Google Scholar]
- 15.Collado P, Jousse-Joulin S, Alcalde M, Naredo E, D’Agostino MA. Is ultrasound a validated imaging tool for the diagnosis and management of synovitis in juvenile idiopathic arthritis? A systemic literature review. Arthritis Care Res. 2012;64(7):1011–9. [DOI] [PubMed] [Google Scholar]
- 16.Magni-Manzoni S, Malattia C, Lanni S, Ravelli A. Advances and challenges in imaging in juvenile idiopathic arthritis. Nat Rev Rheumatol. 2012;8(6):329–36. [DOI] [PubMed] [Google Scholar]
- 17.Roth J. Emergence of musculoskeletal ultrasound use in pediatric rheumatology. Curr Rheumatol Rep. 2020;22(5):14. [DOI] [PubMed] [Google Scholar]
- 18.Doria AS, Kiss MHB, Lotito APN, Molnar LJ, de Castro CC, Medeiros CC, et al. Juvenile rheumatoid arthritis of the knee: evaluation with contrast-enhanced color Doppler ultrasound. Pediatr Radiol. 2001;31(7):524–31. 10.1007/s002470100474. [DOI] [PubMed] [Google Scholar]
- 19.Colebatch-Bourn AN, Edwards CJ, Collado P, D’Agostinor MA, Hemke R, Jousse-Joulin S, et al. EULAR-PReS points to consider for the use of imaging in the diagnosis and management of juvenile idiopathic arthritis in clinical practice. Ann Rheum Dis. 2015;74(11):1946 LP – 1957. Available from: http://ard.bmj.com/content/74/11/1946.abstract. [DOI] [PubMed]
- 20.Lanni S, Martini A, Malattia C. Heading toward a modern imaging approach in juvenile idiopathic arthritis. Curr Rheumatol Rep. 2014;16:416. [DOI] [PubMed] [Google Scholar]
- 21.Damasio M, Malattia C, Martini A, Toma P. Synovial and inflammatory diseases in childhood: role of new imaging modalities in the assessment of patients with juvenile idiopathic arthritis. Pediatr Radiol. 2010;40:985–98. [DOI] [PubMed] [Google Scholar]
- 22.Chang J, Bruns A. Role of musculoskeletal ultrasound in juvenile idiopathic arthritis. Int J Clin Rheumtol. 2013;8(1):97–107. [Google Scholar]
- 23.Tok F, Demirkaya E, Özçakar L. Musculoskeletal ultrasound in pediatric rheumatology. Pediatr Rheumatol. 2011;9(1):25. 10.1186/1546-0096-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vojinovic J, Magni-Manzoni S, Collado P, Windschall D, Ravagnani V, Hernandez-Diaz C, et al. Ultrasonography definitions for synovitis grading in children: the omeract pediatric ultrasound task force. Ann Rheum Dis. 2017;76:1015. [Google Scholar]
- 25.Rossi-Semerano L, Breton S, Semerano L, Boubaya M, Ohanyan H, Bossert M, et al. Application of the OMERACT synovitis ultrasound scoring system in juvenile idiopathic arthritis: a multicenter reliability exercise. Rheumatology (Oxford). 2021;60(8):3579–87. [DOI] [PubMed] [Google Scholar]
- 26.Roth J, Jousse-Joulin S, Magni-Manzoni S, Rodriguez A, Tzaribachev N, Iagnocco A, et al. Definitions for the sonographic features of joints in healthy children. Arthritis Care Res. 2015;67(1):136–42. [DOI] [PubMed] [Google Scholar]
- 27.Windschall D, Collado P, Vojinovic J. Age-related vascularization and ossification of joints in children: an International Pilot Study to Test Multiobserver Ultrasound Reliability. Arthritis Care Res. 2020;72(4):498–506. [DOI] [PubMed] [Google Scholar]
- 28.Spannow AH, Pfeiffer-Jensen M, Andersen NT, Herlin T, Stenbøg E. Ultrasonographic measurements of joint cartilage thickness in healthy children: age- and sex-related standard reference values. J Rheumatol. 2010;37(12):2595–601. [DOI] [PubMed] [Google Scholar]
- 29.Windschall D, Trauzeddel RF, Haller M, Krumrey-Langkammerer M, Nimtz-Talaska A, Berendes R, et al. Pediatric musculoskeletal ultrasound: age- and sex-related normal B-mode findings of the knee. Rheumatol Int. 2016;36(11):1569–77 Available from: 10.1007/s00296-016-3528-x . [DOI] [PubMed] [Google Scholar]
- 30.Chauvin NA, Ho-Fung V, Jaramillo D, Edgar JC, Weiss PF. Ultrasound of the joints and entheses in healthy children. Pediatr Radiol. 2015;45(9):1344–54. 10.1007/s00247-015-3313-0. [DOI] [PubMed] [Google Scholar]
- 31.Windschall D, Malattia C. Ultrasound imaging in paediatric rheumatology. Best Pract Res Clin Rheumatol. 2020;34(6):101570. 10.1016/j.berh.2020.101570. [DOI] [PubMed] [Google Scholar]
- 32.Pascoli L, Wright S, McAllister C, Rooney M. Prospective evaluation of clinical and ultrasound findings in ankle disease in juvenile idiopathic arthritis: importance of ankle ultrasound. J Rheumatol. 2010;37(11):2409 LP – 2414. Available from: http://www.jrheum.org/content/37/11/2409.abstract. [DOI] [PubMed]
- 33.Ventura-Ríos L, Faugier E, Barzola L, De la Cruz-Becerra LB, Sánchez-Bringas G, García AR, et al. Reliability of ultrasonography to detect inflammatory lesions and structural damage in juvenile idiopathic arthritis. Pediatr Rheumatol. 2018;16(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roth J, Ravagnani V, Backhaus M, Balint P, Bruns A, Bruyn GA, et al. Preliminary definitions for the Sonographic Features of Synovitis in Children. Arthritis Care Res. 2017;69(8):1217–23. [DOI] [PubMed] [Google Scholar]
- 35.Collado P, Windschall D, Vojinovic J, Magni-Manzoni S, Balint P, Bruyn GAW, et al. Amendment of the OMERACT Ultrasound definitions of joints’ features in healthy children when using the Doppler technique. Pediatr Rheumatol Online J. 2018;16(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Lucia O, Ravagnani V, Pregnolato F, Hila A, Pontikaki I, Gattinara M, et al. Baseline ultrasound examination as possible predictor of relapse in patients affected by juvenile idiopathic arthritis (JIA). Ann Rheum Dis. 2018;77(10):1426 LP – 1431 Available from: http://ard.bmj.com/content/77/10/1426.abstract . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This manuscript has additional data available as a supplement file.


