Fig 5.
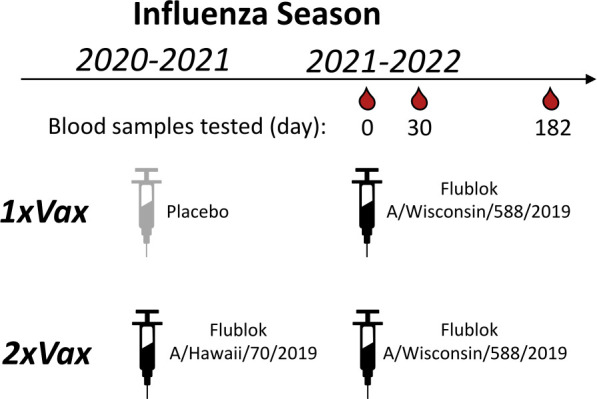
Schematic of study design. In the DRIVE study (29), a randomized vaccine trial of repeated annual vaccination of Flublok (a quadrivalent recombinant-HA vaccine), eligible healthy adults were randomly assigned at enrollment into five intervention groups of receiving a total of 1 to 5 annual Flublok vaccinations during 2020–2021 to 2024–2025 seasons, with blood specimens collected for analysis prior to receipt of the vaccine (day 0) as well as at day 30 and 182 post-vaccination in all participants. For the present analysis, we selected sera collected from study year 2 (2021–2022) from individuals pre-vaccination (day 0) and approximately 30 and 182 days post-vaccination for a subset of participants who had a pre-year 1 vaccination HAI titer <10 against the A/Hawaii/70/2019 (H1N1) vaccine strain, and who were assigned to either receive the placebo or the Flublok Vaccine (A/Hawaii/70/2019) in study year 1 (2020–2021 season). All selected participants received the Flublok Vaccine (A/Wisconsin/588/2019) in year 2 (2021–22 season), that is, two groups of participants who were vaccinated in 2021–2022 and who had either prior year vaccination in 2020–2021 (“2xVax”) or not (“1xVax”) (Table 1).
