FIGURE 4.
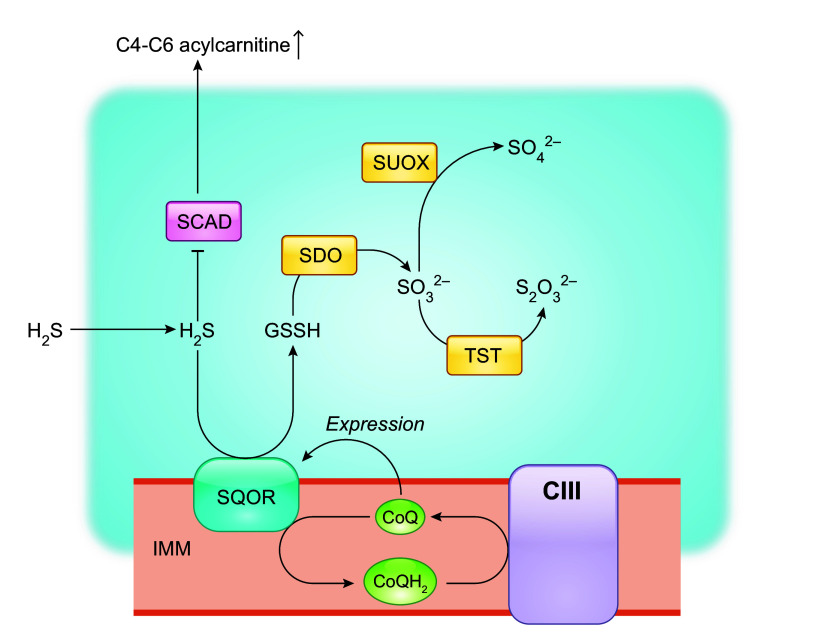
CoQ levels modulate sulfide-quinone oxidoreductase (SQOR) activity. SQOR catalyzes the initial oxidation of hydrogen sulfide (H2S) and utilizes CoQ as the electron acceptor. Sulfur is primarily transferred to glutathione (GSH) under physiological conditions, forming glutathione persulfide (GSSH), which is then oxidized by sulfur dioxygenase (SDO), to produce sulfite () and regenerate GSH. SDO is also known as ethylmalonic encephalopathy protein1 (ETHE1), as its mutations are associated with ethylmalonic encephalopathy, an infantile metabolic disorder. GSSH is also a substrate for thiosulfate sulfurtransferase (TST), which converts to thiosulfate (). Alternatively, is converted to sulfate () by sulfite oxidase (SUOX) residing in the intermembrane space. A further effect of H2S accumulation is inhibition of the enzymatic activity of short-chain acyl-CoA dehydrogenase (SCAD) that catalyzes the first reaction in the β-oxidation of short-chain fatty acids. Elevated blood butyrylcarnitine (C4) is the hallmark biomarker of SCAD deficiency. In addition to acting as a cofactor of SQOR, CoQ levels regulate SQOR transcriptionally by an unknown mechanism. See glossary for other abbreviations.