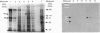Abstract
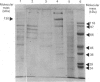
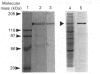
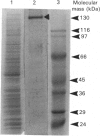
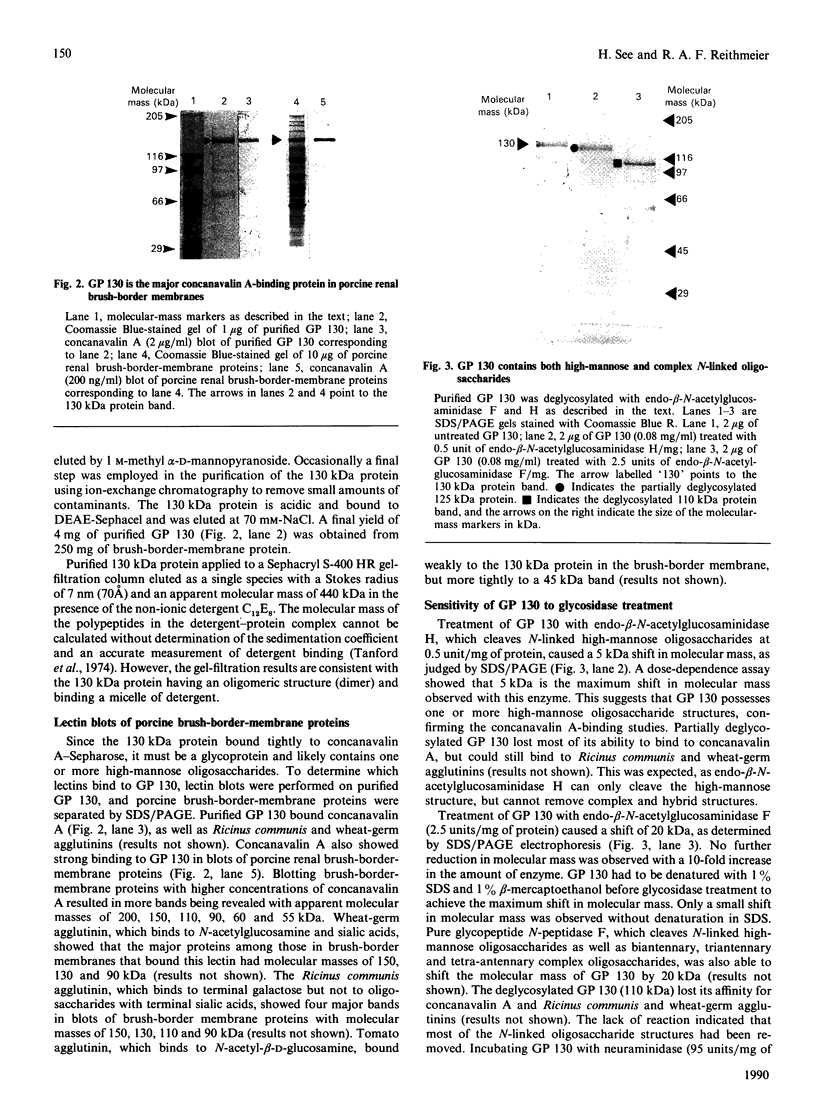
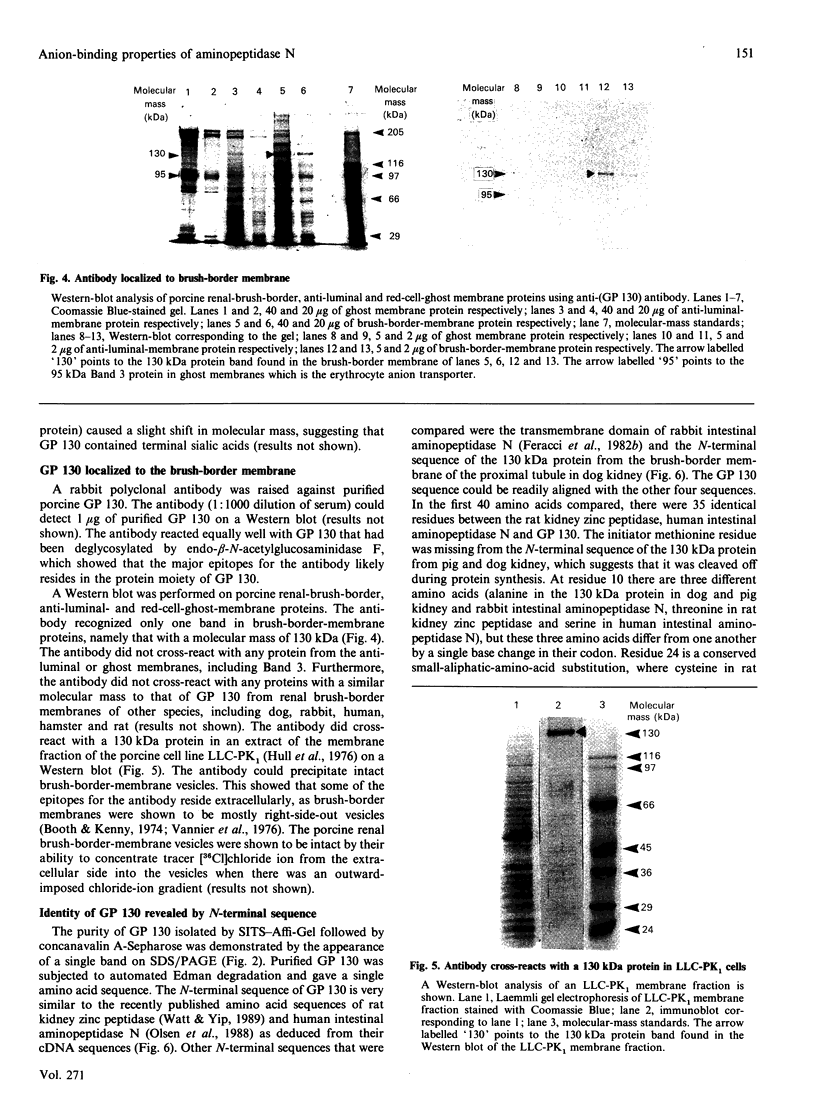
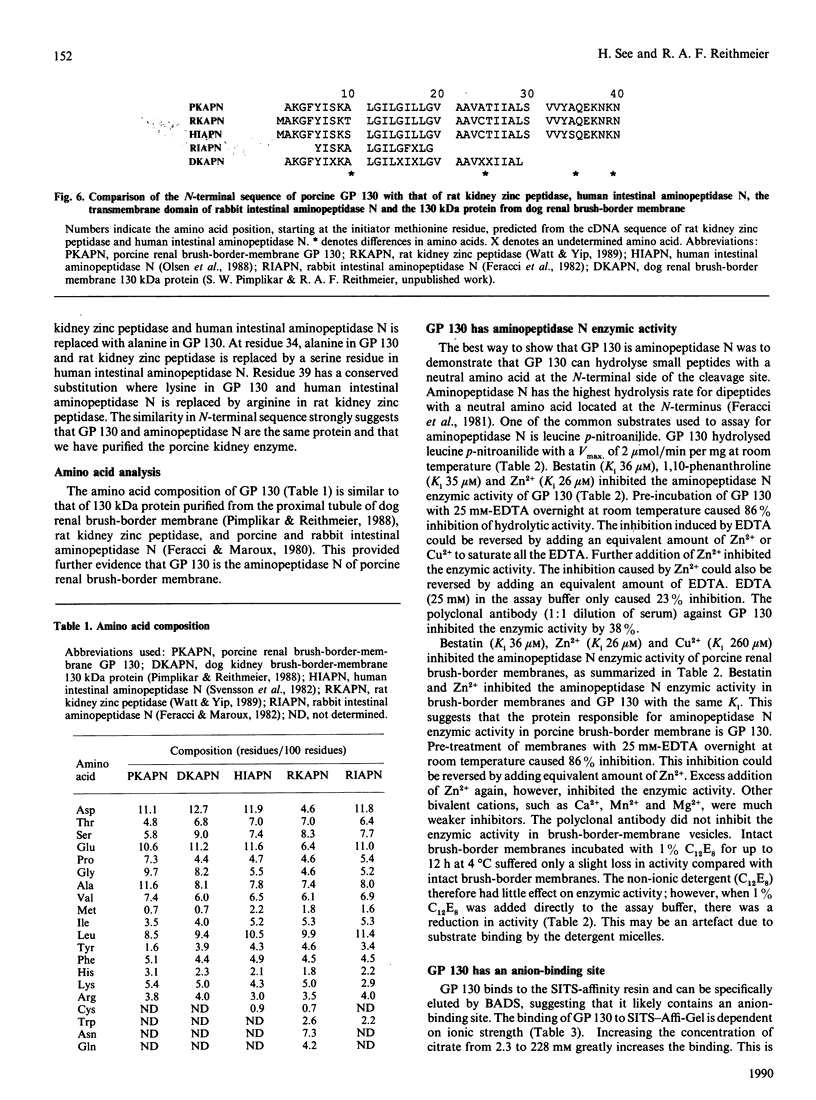
A 130 kDa glycoprotein (GP 130) was purified from porcine renal brush-border membranes by affinity chromatography using immobilized 4-acetamido-4'-isothiocyanostilbene-2,2'-disulphonate (SITS)- and concanavalin A-Sepharose. GP 130 was the major concanavalin A-binding protein in porcine renal brush-border membranes and also bound Ricinus communis (castor-bean) and wheat-germ agglutinins. Endo-beta-N-acetylglucosaminidase F reduced the molecular mass of GP 130 by 20 kDa as determined by SDS/PAGE, whereas endo-beta-N-acetylglucosaminidase H reduced the molecular mass by 5 kDa, showing that GP 130 contained both complex and high-mannose carbohydrate structures. Western-blot analyses using an antibody raised against GP 130 showed that it was localized to the brush-border membrane fraction and was present in a membrane fraction of the pig kidney cell line LLC-PK1. The N-terminal sequence and amino acid composition of GP 130 showed that GP 130 is similar to rat kidney zinc peptidase and human intestinal aminopeptidase N. GP 130 had aminopeptidase N enzymic activity and was inhibited by bestatin (Ki = 36 microM), 1,10-phenanthroline (Ki 30 microM), Zn2+ (Ki 26 microM), Cu2+ (Ki 260 microM), pre-incubation with EDTA and by a polyclonal antibody against GP 130. Bicarbonate and iodide blocked the binding of GP 130 to the SITS-affinity resin, showing that GP 130 has an anion-binding site. Neither these anions nor stilbene disulphonates affected the aminopeptidase N activity of GP 130.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson P. S. The renal proximal tubule: a model for diversity of anion exchangers and stilbene-sensitive anion transporters. Annu Rev Physiol. 1989;51:419–441. doi: 10.1146/annurev.ph.51.030189.002223. [DOI] [PubMed] [Google Scholar]
- Booth A. G., Hubbard L. M., Kenny A. J. Proteins of the kidney microvillar membrane. Immunoelectrophoretic analysis of the membrane hydrolases: identification and resolution of the detergent- and proteinase-solubilized forms. Biochem J. 1979 May 1;179(2):397–405. doi: 10.1042/bj1790397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Danielsen E. M., Norén O., Sjöström H. Biosynthesis of intestinal microvillar proteins. Translational evidence in vitro that aminopeptidase N is synthesized as a Mr-115000 polypeptide. Biochem J. 1982 Apr 15;204(1):323–327. doi: 10.1042/bj2040323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feracci H., Benajiba A., Gorvel J. P., Doumeng C., Maroux S. Enzymatic and immunological properties of the protease form of aminopeptidase N and A from pig and rabbit intestinal brush border. Biochim Biophys Acta. 1981 Mar 13;658(1):148–157. doi: 10.1016/0005-2744(81)90258-8. [DOI] [PubMed] [Google Scholar]
- Feracci H., Bernadac A., Gorvel J. P., Maroux S. Localization by immunofluorescence and histochemical labeling of aminopeptidase N in relation to its biosynthesis in rabbit and pig enterocytes. Gastroenterology. 1982 Feb;82(2):317–324. [PubMed] [Google Scholar]
- Feracci H., Maroux S., Bonicel J., Desnuelle P. The amino acid sequence of the hydrophobic anchor of rabbit intestinal brush border aminopeptidase N. Biochim Biophys Acta. 1982 Jan 4;684(1):133–136. doi: 10.1016/0005-2736(82)90057-8. [DOI] [PubMed] [Google Scholar]
- Feracci H., Maroux S. Rabbit intestinal aminopeptidase N. Purification and molecular properties. Biochim Biophys Acta. 1980 Jul;599(2):448–463. doi: 10.1016/0005-2736(80)90190-x. [DOI] [PubMed] [Google Scholar]
- Grassl S. M., Holohan P. D., Ross C. R. HCO3- transport in basolateral membrane vesicles isolated from rat renal cortex. J Biol Chem. 1987 Feb 25;262(6):2682–2687. [PubMed] [Google Scholar]
- Hirose J., Ando S., Kidani Y. Excess zinc ions are a competitive inhibitor for carboxypeptidase A. Biochemistry. 1987 Oct 6;26(20):6561–6565. doi: 10.1021/bi00394a041. [DOI] [PubMed] [Google Scholar]
- Hirota T., Nishikawa Y., Takahagi H., Igarashi T., Kitagawa H. Simultaneous purification and properties of dehydropeptidase-I and aminopeptidase-M from rat kidney. Res Commun Chem Pathol Pharmacol. 1985 Sep;49(3):435–445. [PubMed] [Google Scholar]
- Hull R. N., Cherry W. R., Weaver G. W. The origin and characteristics of a pig kidney cell strain, LLC-PK. In Vitro. 1976 Oct;12(10):670–677. doi: 10.1007/BF02797469. [DOI] [PubMed] [Google Scholar]
- Ishiura S., Yamamoto T., Yamamoto M., Nojima M., Aoyagi T., Sugita H. Human skeletal muscle contains two major aminopeptidases: an anion-activated aminopeptidase B and an aminopeptidase M-like enzyme. J Biochem. 1987 Nov;102(5):1023–1031. doi: 10.1093/oxfordjournals.jbchem.a122140. [DOI] [PubMed] [Google Scholar]
- Jennings M. L., Passow H. Anion transport across the erythrocyte membrane, in situ proteolysis of band 3 protein, and cross-linking of proteolytic fragments by 4,4'-diisothiocyano dihydrostilbene-2,2'-disulfonate. Biochim Biophys Acta. 1979 Jul 5;554(2):498–519. doi: 10.1016/0005-2736(79)90387-0. [DOI] [PubMed] [Google Scholar]
- Karniski L. P., Aronson P. S. Anion exchange pathways for Cl- transport in rabbit renal microvillus membranes. Am J Physiol. 1987 Sep;253(3 Pt 2):F513–F521. doi: 10.1152/ajprenal.1987.253.3.F513. [DOI] [PubMed] [Google Scholar]
- Karniski L. P., Aronson P. S. Chloride/formate exchange with formic acid recycling: a mechanism of active chloride transport across epithelial membranes. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6362–6365. doi: 10.1073/pnas.82.18.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf P. A., Rothstein A. Chemical modification of membranes. I. Effects of sulfhydryl and amino reactive reagents on anion and cation permeability of the human red blood cell. J Gen Physiol. 1971 Aug;58(2):190–210. doi: 10.1085/jgp.58.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotaki A., Naoi M., Yagi K. A diaminostilbene dye as a hydrophobic probe for proteins. Biochim Biophys Acta. 1971 Mar 23;229(3):547–556. doi: 10.1016/0005-2795(71)90270-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehky P., Lisowski J., Wolf D. P., Wacker H., Stein E. A. Pig kidney particulate aminopeptidase. A zinc metalloenzyme. Biochim Biophys Acta. 1973 Sep 15;321(1):274–281. doi: 10.1016/0005-2744(73)90082-x. [DOI] [PubMed] [Google Scholar]
- Lowe A. G., Lambert A. Chloride-bicarbonate exchange and related transport processes. Biochim Biophys Acta. 1982 Dec;694(4):353–374. doi: 10.1016/0304-4157(82)90002-8. [DOI] [PubMed] [Google Scholar]
- Maroux S., Louvard D., Baratti J. The aminopeptidase from hog intestinal brush border. Biochim Biophys Acta. 1973 Sep 15;321(1):282–295. doi: 10.1016/0005-2744(73)90083-1. [DOI] [PubMed] [Google Scholar]
- McDermott J. R., Mantle D., Lauffart B., Gibson A. M., Biggins J. A. Purification and characterization of two soluble Cl(-)-activated arginyl aminopeptidases from human brain and their endopeptidase action on neuropeptides. J Neurochem. 1988 Jan;50(1):176–182. doi: 10.1111/j.1471-4159.1988.tb13246.x. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Olsen J., Cowell G. M., Kønigshøfer E., Danielsen E. M., Møller J., Laustsen L., Hansen O. C., Welinder K. G., Engberg J., Hunziker W. Complete amino acid sequence of human intestinal aminopeptidase N as deduced from cloned cDNA. FEBS Lett. 1988 Oct 10;238(2):307–314. doi: 10.1016/0014-5793(88)80502-7. [DOI] [PubMed] [Google Scholar]
- Pimplikar S. W., Reithmeier R. A. Affinity chromatography of Band 3, the anion transport protein of erythrocyte membranes. J Biol Chem. 1986 Jul 25;261(21):9770–9778. [PubMed] [Google Scholar]
- Pimplikar S. W., Reithmeier R. A. Identification, purification, and characterization of a stilbenedisulfonate binding glycoprotein from canine kidney brush border membranes. A candidate for a renal anion exchanger. J Biol Chem. 1988 Mar 25;263(9):4485–4493. [PubMed] [Google Scholar]
- Ramjeesingh M., Grinstein S., Rothstein A. Intrinsic segments of band 3 that are associated with anion transport across red blood cell membranes. J Membr Biol. 1980 Dec 15;57(2):95–102. doi: 10.1007/BF01868996. [DOI] [PubMed] [Google Scholar]
- Sacktor B., Rosenbloom I. L., Liang C. T., Cheng L. Sodium gradient- and sodium plus potassium gradient-dependent L-glutamate uptake in renal basolateral membrane vesicles. J Membr Biol. 1981 May 15;60(1):63–71. doi: 10.1007/BF01870833. [DOI] [PubMed] [Google Scholar]
- Stewart J. R., Kenny A. J. Proteins of the kidney microvillar membrane. Biosynthesis of endopeptidase-24.11, dipeptidylpeptidase IV and aminopeptidases N and A in pig kidney slices. Biochem J. 1984 Dec 1;224(2):549–558. doi: 10.1042/bj2240549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson B., Sjöström H., Norén O. Structural studies on microvillus aminopeptidase from pig small intestine. Eur J Biochem. 1982 Sep 1;126(3):481–488. doi: 10.1111/j.1432-1033.1982.tb06805.x. [DOI] [PubMed] [Google Scholar]
- Tanford C., Nozaki Y., Reynolds J. A., Makino S. Molecular characterization of proteins in detergent solutions. Biochemistry. 1974 May 21;13(11):2369–2376. doi: 10.1021/bi00708a021. [DOI] [PubMed] [Google Scholar]
- Vannier C., Louvard D., Maroux S., Desnuelle P. Structural and topological homology between porcine intestinal and renal brush border aminopeptidase. Biochim Biophys Acta. 1976 Nov 11;455(1):185–199. doi: 10.1016/0005-2736(76)90163-2. [DOI] [PubMed] [Google Scholar]
- Wacker H., Lehky P., Fischer E. H., Stein E. A. Physical and chemical characterization of pig kidney particulate aminopeptidase. Helv Chim Acta. 1971;54(2):473–485. doi: 10.1002/hlca.19710540206. [DOI] [PubMed] [Google Scholar]
- Watt V. M., Yip C. C. Amino acid sequence deduced from a rat kidney cDNA suggests it encodes the Zn-peptidase aminopeptidase N. J Biol Chem. 1989 Apr 5;264(10):5480–5487. [PubMed] [Google Scholar]
- Yamashita K., Tachibana Y., Matsuda Y., Katunuma N., Kochibe N., Kobata A. Comparative studies of the sugar chains of aminopeptidase N and dipeptidylpeptidase IV purified from rat kidney brush-border membrane. Biochemistry. 1988 Jul 26;27(15):5565–5573. doi: 10.1021/bi00415a026. [DOI] [PubMed] [Google Scholar]