FIGURE 1.
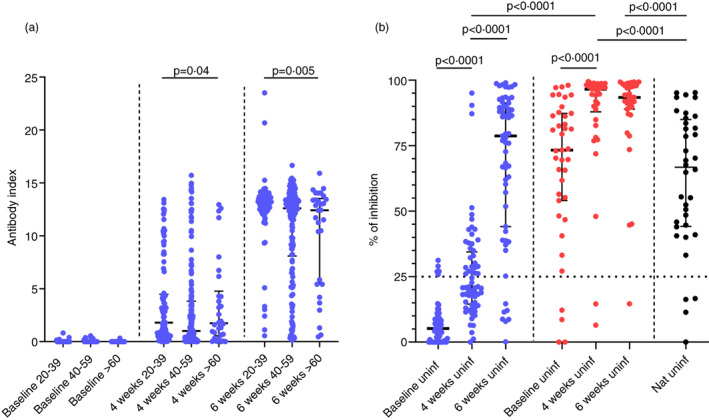
SARS‐CoV‐2 specific antibody responses (total antibodies and ACE2 receptor blocking antibodies in those who received the Sinopharm/BBIBP‐CorV vaccine. (a) SARS‐CoV‐2 specific total antibodies were measured by ELISA in 20‐ to 39‐year‐old individuals (n = 89), 40‐ to 59‐year olds (n = 163) and >60 years olds (n = 30) at 4 weeks following the first dose and again at 6 weeks (2 weeks after the second dose). (b) ACE2 receptor blocking antibodies were measured by the surrogate neutralizing antibody assay in a sub cohort of these individuals (n = 64) at 4 and 6 weeks, in a cohort of individuals (n = 38), who were found to be seropositive for SARS‐CoV‐2 at the time of recruitment and in a cohort of naturally infected individuals 6 weeks following onset of illness (n = 36). The differences in the total antibody titres between different age groups was determined by the Kruskal–Wallis test. the differences in ACE2 receptor blocking antibodies at baseline uninfected and infected individuals were assessed by the Wilcoxon matched‐pairs signed ranked test. all tests were two sided. the error bars indicate the median and the interquartile ranges. Baseline uninfected individuals: Blue, baseline infected individuals: Red, naturally infected unvaccinated individuals: Black
