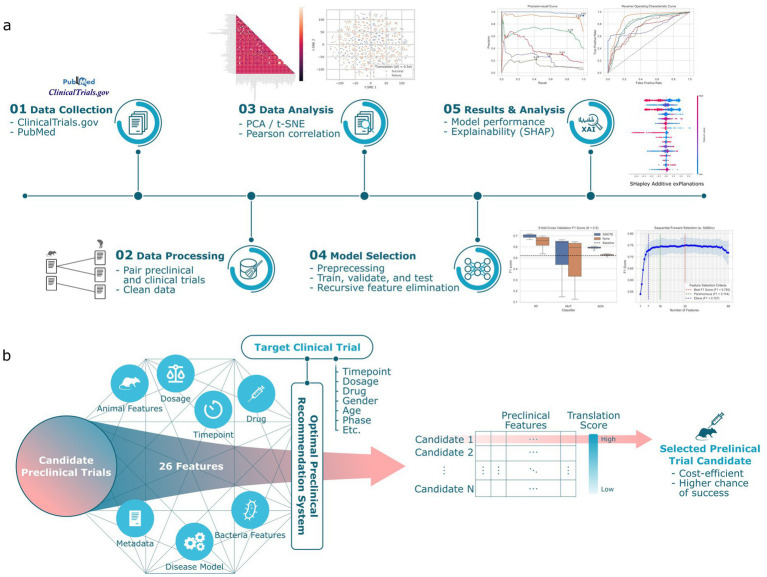
Figure 1.
Overview of the preclinical recommendation system. (a) We collect data from publicly available preclinical and clinical trial information about Clostridium difficile infection. This dataset, designated as A2H, is constructed by pairing the preclinical trial with the clinical trial that shares the same drug. A binary classification label is applied to each pair, where a translation is successful (label 1) if the preclinical survival rates and clinical recovery rates are within a threshold . Then, a machine learning pipeline chooses the best combination of feature selection, missing value imputation, outlier detection, and classifier. We report the model performance and feature interpretation and predictions. (b) For any specified clinical trial of interest, our system computes a translation score for each candidate preclinical trial. This score quantitatively assesses the potential for successful translation. The preclinical trial that emerges with the highest translation score is then preferentially chosen to inform the design of the ensuing preclinical study.