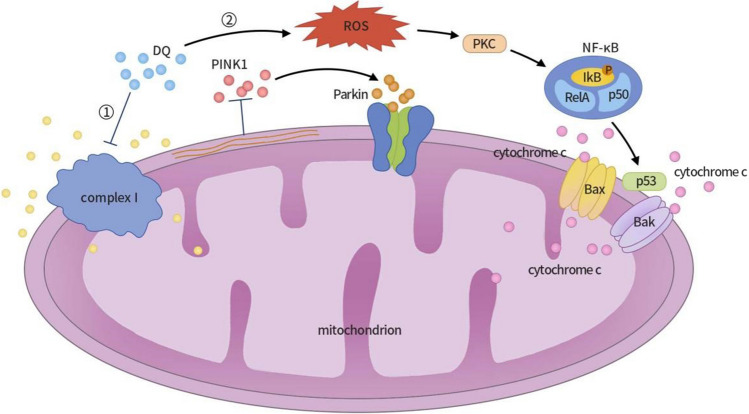
Fig. 3.
Mechanisms of DQ-induced neurotoxicity and apoptosis in PC12 nerve cells. (1) When PC12 nerve cells are exposed to a DQ solution, DQ inhibits mitochondrial complex I activity, causing changes in mitochondrial membrane potential and inhibiting PINK1 introduction to mitochondria. The accumulation of PINK1 triggers the activation and recruitment of Parkin protein, promoting the ubiquitination and degradation of mitochondrial outer membrane proteins, ultimately leading to the autophagic clearance of damaged mitochondria. (2) Reactive oxygen species (ROS) activate protein kinase C (PKC), which, in turn, phosphorylates protein IκB, leading to the dissociation of IκB from NF-κB and the subsequent activation of NF-κB. NF-κB regulates the expression of the pro-apoptotic factor P53, enhancing its expression in PC12 nerve cells and causing its accumulation in the cytoplasm and nucleus. When stress-induced damage becomes irreversible, P53 translocates to the mitochondrial outer membrane, triggering the release of mitochondrial pro-apoptotic genes Bax and Bak. Bax and Bak can form mitochondrial permeability transition pores, releasing cytochrome C, exacerbating mitochondrial dysfunction, and ultimately resulting in the apoptosis of PC12 nerve cells. DQ, diquat; PINK1, PTEN-induced putative kinase 1; ROS, reactive oxygen species; PKC, protein kinase C; NF-κB, nuclear factor-kappa B