Abstract
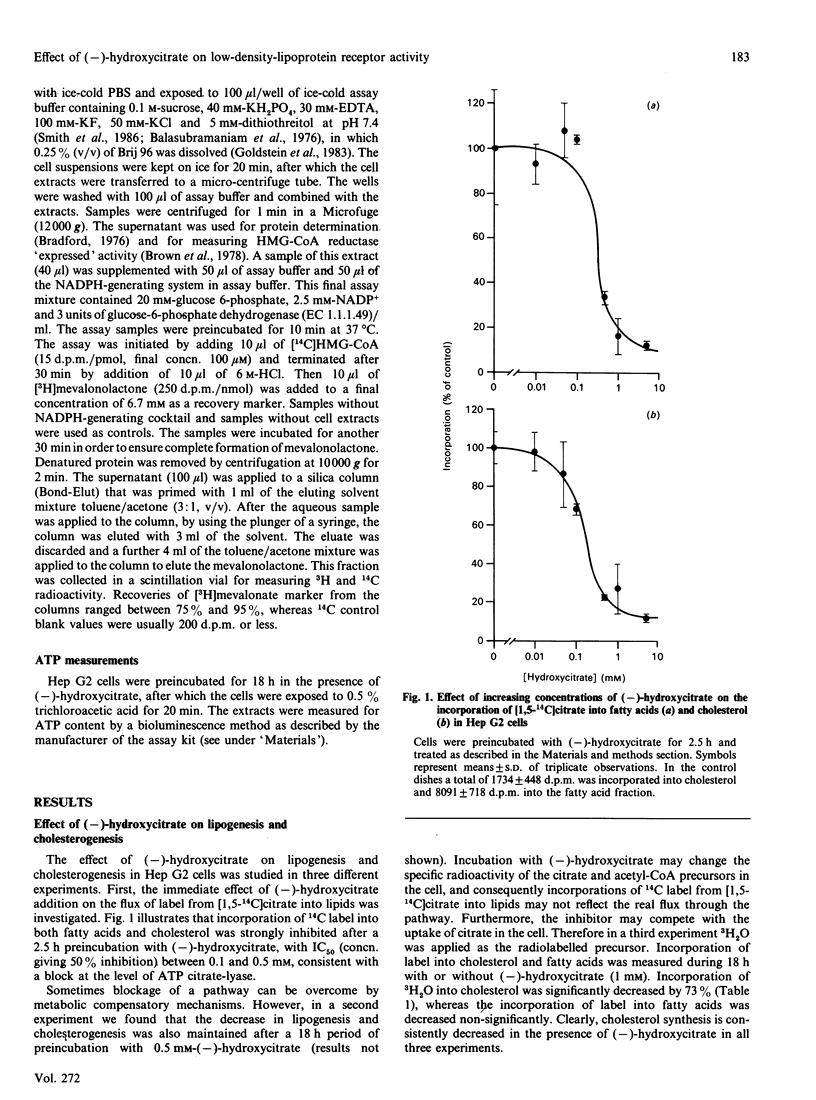
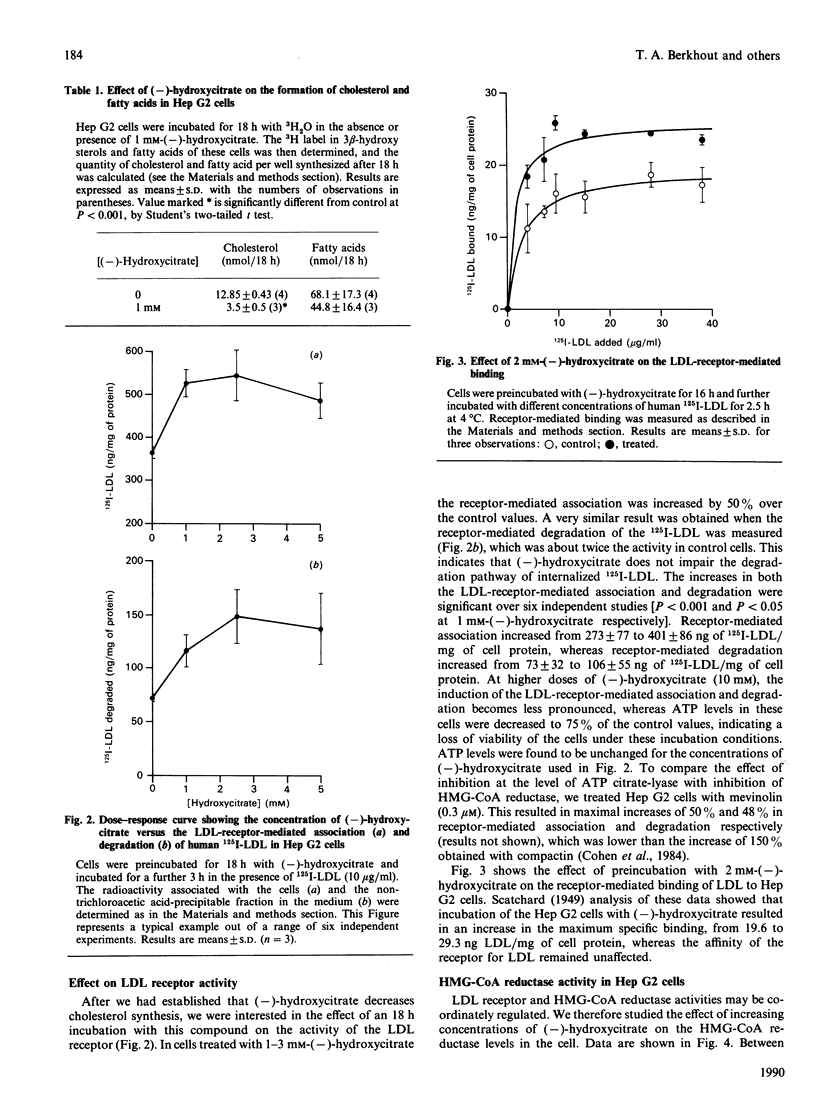
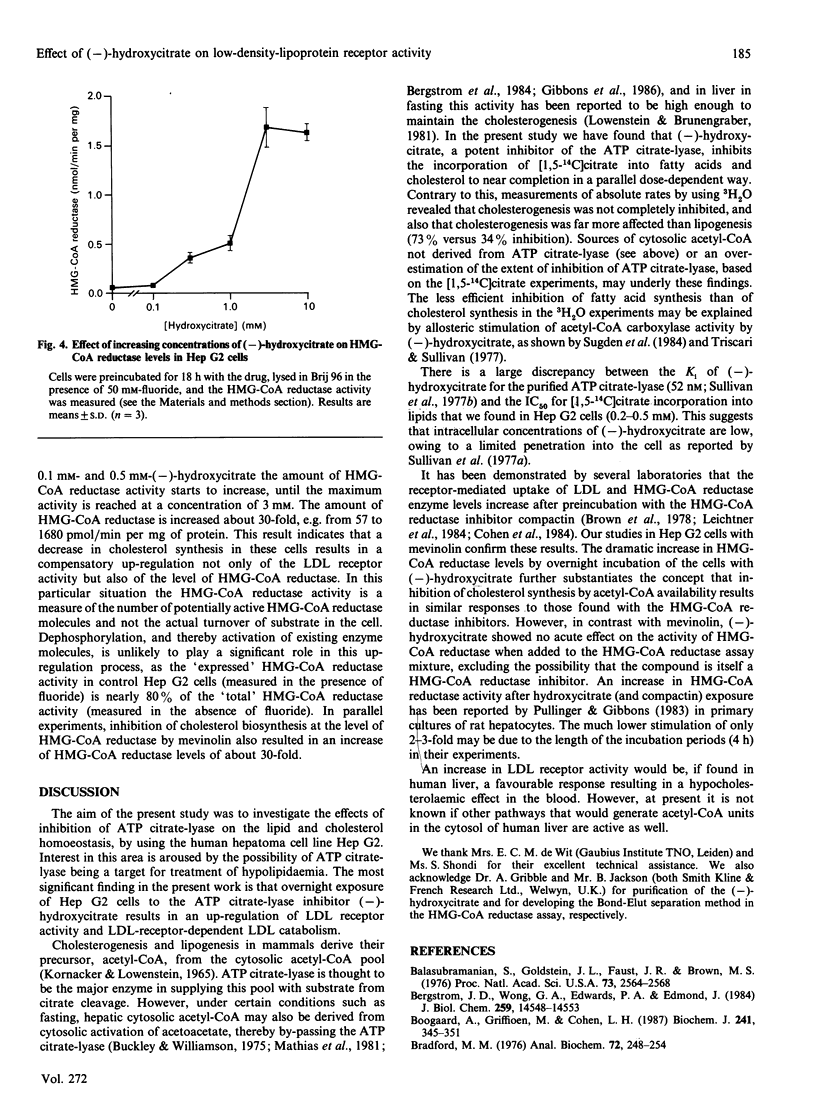
(-)-Hydroxycitrate, a potent inhibitor of ATP citrate-lyase, was tested in Hep G2 cells for effects on cholesterol homoeostasis. After 2.5 h and 18 h incubations with (-)-hydroxycitrate at concentrations of 0.5 mM or higher, incorporation of [1,5-14C]citrate into fatty acids and cholesterol was strongly inhibited. This most likely reflects an effective inhibition of ATP citrate-lyase. Cholesterol biosynthesis was decreased to 27% of the control value as measured by incorporations from 3H2O, indicating a decreased flux of carbon units through the cholesterol-synthetic pathway. After 18 h preincubation with 2 mM-(-)-hydroxycitrate, the cellular low-density-lipoprotein (LDL) receptor activity was increased by 50%, as determined by the receptor-mediated association and degradation. Measurements of receptor-mediated binding versus LDL concentration suggests that this increase was due to an increase in the numbers of LDL receptors. Simultaneously, enzyme levels of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase as determined by activity measurements increased 30-fold. Our results suggest that the increases in HMG-CoA reductase and the LDL receptor are initiated by the decreased flux of carbon units in the cholesterol-synthetic pathway, owing to inhibition of ATP citratelyase. A similar induction of HMG-CoA reductase and LDL receptor was also found after preincubations of cells with 0.3 microM-mevinolin, suggesting that the underlying mechanism for this induction is identical for both drugs.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balasubramaniam S., Goldstein J. L., Faust J. R., Brown M. S. Evidence for regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and cholesterol synthesis in nonhepatic tissues of rat. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2564–2568. doi: 10.1073/pnas.73.8.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom J. D., Wong G. A., Edwards P. A., Edmond J. The regulation of acetoacetyl-CoA synthetase activity by modulators of cholesterol synthesis in vivo and the utilization of acetoacetate for cholesterogenesis. J Biol Chem. 1984 Dec 10;259(23):14548–14553. [PubMed] [Google Scholar]
- Boogaard A., Griffioen M., Cohen L. H. Regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase in human hepatoma cell line Hep G2. Effects of inhibitors of cholesterol synthesis on enzyme activity. Biochem J. 1987 Jan 15;241(2):345–351. doi: 10.1042/bj2410345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Faust J. R., Goldstein J. L., Kaneko I., Endo A. Induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts incubated with compactin (ML-236B), a competitive inhibitor of the reductase. J Biol Chem. 1978 Feb 25;253(4):1121–1128. [PubMed] [Google Scholar]
- Buckley B. M., Williamson D. H. Acetoacetyl-CoA synthetase; a lipogenic enzyme in rat tissues. FEBS Lett. 1975 Dec 1;60(1):7–10. doi: 10.1016/0014-5793(75)80406-6. [DOI] [PubMed] [Google Scholar]
- Cheema-Dhadli S., Halperin M. L., Leznoff C. C. Inhibition of enzymes which interact with citrate by (--)hydroxycitrate and 1,2,3,-tricarboxybenzene. Eur J Biochem. 1973 Sep 21;38(1):98–102. doi: 10.1111/j.1432-1033.1973.tb03038.x. [DOI] [PubMed] [Google Scholar]
- Cohen L. H., Griffioen M., Havekes L., Schouten D., van Hinsbergh V., Kempen H. J. Effects of compactin, mevalonate and low-density lipoprotein on 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity and low-density-lipoprotein-receptor activity in the human hepatoma cell line Hep G2. Biochem J. 1984 Aug 15;222(1):35–39. doi: 10.1042/bj2220035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson P. A., Van der Westhuyzen D. R., Goldstein J. L., Brown M. S. Purification of oxysterol binding protein from hamster liver cytosol. J Biol Chem. 1989 May 25;264(15):9046–9052. [PubMed] [Google Scholar]
- Dietschy J. M., Spady D. K. Measurement of rates of cholesterol synthesis using tritiated water. J Lipid Res. 1984 Dec 15;25(13):1469–1476. [PubMed] [Google Scholar]
- Endo A., Kuroda M., Tanzawa K. Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett. 1976 Dec 31;72(2):323–326. doi: 10.1016/0014-5793(76)80996-9. [DOI] [PubMed] [Google Scholar]
- Gibbons G. F., Attwell Thomas C. P., Pullinger C. R. The metabolic route by which oleate is converted into cholesterol in rat hepatocytes. Biochem J. 1986 Apr 1;235(1):19–24. doi: 10.1042/bj2350019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons G. F., Pullinger C. R., Munday M. R., Williamson D. H. Regulation of cholesterol synthesis in the liver and mammary gland of the lactating rat. Biochem J. 1983 Jun 15;212(3):843–848. doi: 10.1042/bj2120843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brown M. S. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- Hamilton J. G., Sullivan A. C., Kritchevsky D. Hupolipidemic activity of (--)-hydroxycitrate. Lipids. 1977 Jan;12(1):1–9. doi: 10.1007/BF02532964. [DOI] [PubMed] [Google Scholar]
- Havekes L. M., Schouten D., de Wit E. C., Cohen L. H., Griffioen M., van Hinsbergh V. W., Princen H. M. Stimulation of the LDL receptor activity in the human hepatoma cell line Hep G2 by high-density serum fractions. Biochim Biophys Acta. 1986 Feb 12;875(2):236–246. doi: 10.1016/0005-2760(86)90173-6. [DOI] [PubMed] [Google Scholar]
- Havekes L., van Hinsbergh V., Kempen H. J., Emeis J. The metabolism in vitro of human low-density lipoprotein by the human hepatoma cell line Hep G2. Biochem J. 1983 Sep 15;214(3):951–958. doi: 10.1042/bj2140951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel C., Hansbury E., Scallen T. J., Watson J. A. Regulation of cholesterol synthesis in primary rat hepatocyte culture cells. Possible regulatory site at sterol demethylation. J Biol Chem. 1979 Oct 10;254(19):9573–9582. [PubMed] [Google Scholar]
- Jungas R. L. Fatty acid synthesis in adipose tissue incubated in tritiated water. Biochemistry. 1968 Oct;7(10):3708–3717. doi: 10.1021/bi00850a050. [DOI] [PubMed] [Google Scholar]
- KORNACKER M. S., LOWENSTEIN J. M. CITRATE AND THE CONVERSION OF CARBOHYDRATE INTO FAT. THE ACTIVITIES OF CITRATE-CLEAVAGE ENZYME AND ACETATE THIOKINASE IN LIVERS OF STARVED AND RE-FED RATS. Biochem J. 1965 Jan;94:209–215. doi: 10.1042/bj0940209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluzny M. A., Duncan L. A., Merritt M. V., Epps D. E. Rapid separation of lipid classes in high yield and purity using bonded phase columns. J Lipid Res. 1985 Jan;26(1):135–140. [PubMed] [Google Scholar]
- Kempen H. J., van Son K., Cohen L. H., Griffioen M., Verboom H., Havekes L. Effect of ketoconazole on cholesterol synthesis and on HMG-CoA reductase and LDL-receptor activities in Hep G2 cells. Biochem Pharmacol. 1987 Apr 15;36(8):1245–1249. doi: 10.1016/0006-2952(87)90077-3. [DOI] [PubMed] [Google Scholar]
- Kovanen P. T., Bilheimer D. W., Goldstein J. L., Jaramillo J. J., Brown M. S. Regulatory role for hepatic low density lipoprotein receptors in vivo in the dog. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1194–1198. doi: 10.1073/pnas.78.2.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leichtner A. M., Krieger M., Schwartz A. L. Regulation of low density lipoprotein receptor function in a human hepatoma cell line. Hepatology. 1984 Sep-Oct;4(5):897–901. doi: 10.1002/hep.1840040518. [DOI] [PubMed] [Google Scholar]
- Lowenstein J. M., Brunengraber H. Hydroxycitrate. Methods Enzymol. 1981;72:486–497. doi: 10.1016/s0076-6879(81)72038-x. [DOI] [PubMed] [Google Scholar]
- Lowenstein J. M. Effect of (-)-hydroxycitrate on fatty acid synthesis by rat liver in vivo. J Biol Chem. 1971 Feb 10;246(3):629–632. [PubMed] [Google Scholar]
- Mathias M. M., Sullivan A. C., Hamilton J. G. Fatty acid and cholesterol synthesis from specifically labeled leucine by isolated rat hepatocytes. Lipids. 1981 Oct;16(10):739–743. doi: 10.1007/BF02535341. [DOI] [PubMed] [Google Scholar]
- Osborne T. F., Gil G., Goldstein J. L., Brown M. S. Operator constitutive mutation of 3-hydroxy-3-methylglutaryl coenzyme A reductase promoter abolishes protein binding to sterol regulatory element. J Biol Chem. 1988 Mar 5;263(7):3380–3387. [PubMed] [Google Scholar]
- Panini S. R., Sexton R. C., Gupta A. K., Parish E. J., Chitrakorn S., Rudney H. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and cholesterol biosynthesis by oxylanosterols. J Lipid Res. 1986 Nov;27(11):1190–1204. [PubMed] [Google Scholar]
- Pullinger C. R., Gibbons G. F. The role of substrate supply in the regulation of cholesterol biosynthesis in rat hepatocytes. Biochem J. 1983 Mar 15;210(3):625–632. doi: 10.1042/bj2100625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave T. G., Roberts D. C., West C. E. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem. 1975 May 12;65(1-2):42–49. doi: 10.1016/0003-2697(75)90488-1. [DOI] [PubMed] [Google Scholar]
- Rudney H., Sexton R. C. Regulation of cholesterol biosynthesis. Annu Rev Nutr. 1986;6:245–272. doi: 10.1146/annurev.nu.06.070186.001333. [DOI] [PubMed] [Google Scholar]
- Smith R. A., Middleton B., West D. W. Diurnal variation in the fraction of 3-hydroxy-3-methylglutaryl-coenzyme A reductase in the active form in the mammary gland of the lactating rat. Biochem J. 1986 Oct 15;239(2):285–293. doi: 10.1042/bj2390285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden M. C., Watts D. I., West P. S., Palmer T. N. Proline and hepatic lipogenesis. Biochim Biophys Acta. 1984 Apr 24;798(3):368–373. doi: 10.1016/0304-4165(84)90111-9. [DOI] [PubMed] [Google Scholar]
- Sullivan A. C., Hamilton J. G., Miller O. N., Wheatley V. R. Inhibition of lipogenesis in rat liver by (-)-hydroxycitrate. Arch Biochem Biophys. 1972 May;150(1):183–190. doi: 10.1016/0003-9861(72)90025-2. [DOI] [PubMed] [Google Scholar]
- Sullivan A. C., Singh M., Srere P. A., Glusker J. P. Reactivity and inhibitor potential of hydroxycitrate isomers with citrate synthase, citrate lyase, and ATP citrate lyase. J Biol Chem. 1977 Nov 10;252(21):7583–7590. [PubMed] [Google Scholar]
- Südhof T. C., Russell D. W., Brown M. S., Goldstein J. L. 42 bp element from LDL receptor gene confers end-product repression by sterols when inserted into viral TK promoter. Cell. 1987 Mar 27;48(6):1061–1069. doi: 10.1016/0092-8674(87)90713-6. [DOI] [PubMed] [Google Scholar]
- Triscari J., Sullivan A. C. Comparative effects of (--)-hydroxycitrate and (+)-allo-hydroxycitrate on acetyl CoA carboxylase and fatty acid and cholesterol synthesis in vivo. Lipids. 1977 Apr;12(4):357–363. doi: 10.1007/BF02533638. [DOI] [PubMed] [Google Scholar]
- Wang S. R., Pessah M., Infante J., Catala D., Salvat C., Infante R. Lipid and lipoprotein metabolism in Hep G2 cells. Biochim Biophys Acta. 1988 Aug 12;961(3):351–363. doi: 10.1016/0005-2760(88)90082-3. [DOI] [PubMed] [Google Scholar]
- Watson J. A., Fang M., Lowenstein J. M. Tricarballylate and hydroxycitrate: substrate and inhibitor of ATP: citrate oxaloacetate lyase. Arch Biochem Biophys. 1969 Dec;135(1):209–217. doi: 10.1016/0003-9861(69)90532-3. [DOI] [PubMed] [Google Scholar]


