Figure 6.
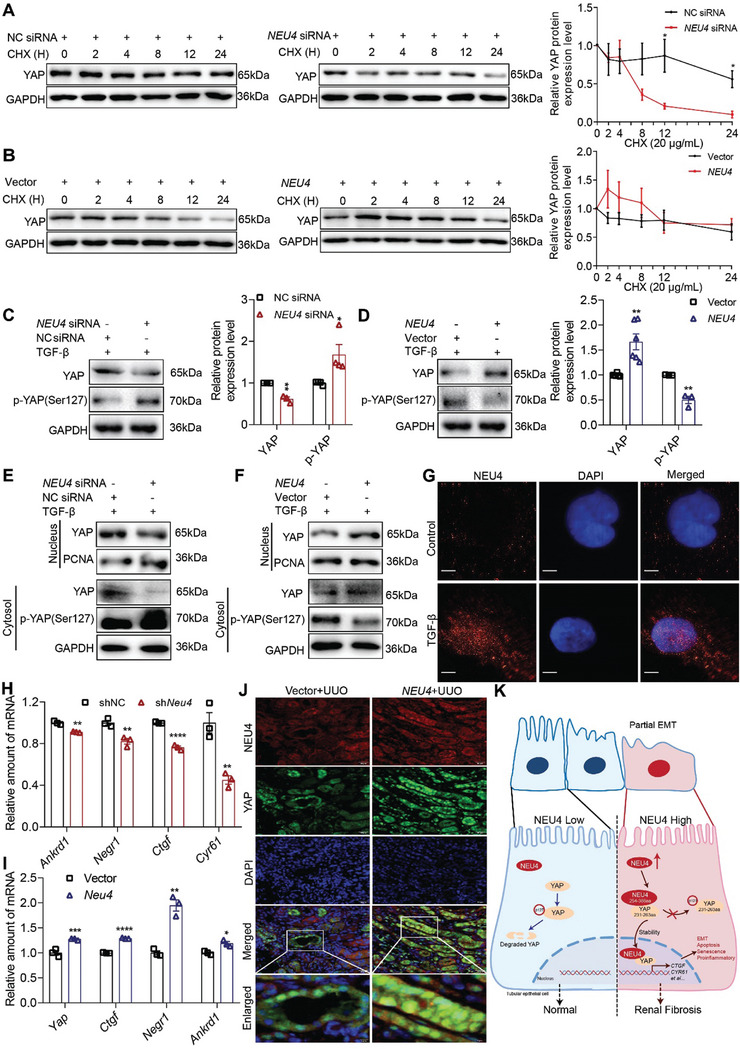
NEU4 inhibited activation of YAP. A,B) Western blot (left panel) and quantification (right panel) of the protein expression of YAP in HK‐2 cells treatment with TGF‐β for 24 h, and then with cycloheximide (CHX, 20 µg mL−1) for the indicated periods of time (0, 2, 4, 8, 12, 24 h) after transfection either with NEU4 siRNA (A) or NEU4 overexpression plasmid (B). GAPDH served as loading control. n = 3 biologically independent samples. C,D) Western blot (left panel) and quantification (right panel) of the protein expression of YAP and phosphorylation of YAP in HK‐2 cells treatment with TGF‐β 24 h after transfection either NEU4 siRNA (C) or NEU4 overexpression plasmid (D). GAPDH served as loading control. n = 3–6 samples. E,F) Western blot of YAP and phosphorylation of YAP in nuclear and cytosol of HK‐2 cells following 24 h after treatment with TGF‐β subsequent to transfection with either NEU4 siRNA (E) or NEU4 overexpression plasmid (F). G) Localization of NEU4 was analyzed by immunofluorescence in HK‐2 cells stimulated with TGF‐β for 24 h. Scale bar, 5 µm. H,I) mRNA abundance of Yap target genes in left kidney tissue of UUO‐mice treated with shNeu4 (H) or Neu4 overexpression plasmid (I). n = 3 mice. J) Colocalization of NEU4 and YAP was analyzed by immunofluorescence in left kidneys. Scale bar, 50 µm. K) The proposed mechanisms of NEU4‐mediated renal fibrosis. Error bars represent mean ± SEM. Comparisons between two groups were analyzed by using a two‐tailed Studentʹs t test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 versus the NC siRNA, shNC or Vector group.
