Fig. 1.
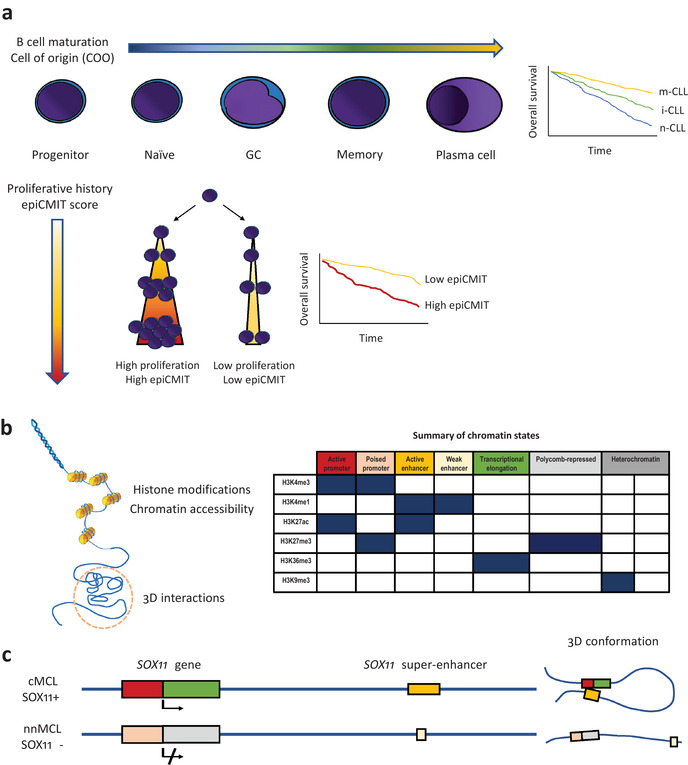
Epigenetics in B‐cell malignancies. (a) DNA methylation changes during B‐cell maturation (upper panel); in B‐cell neoplasms, DNA methylation imprints of normal B‐cell maturation allow classification of them into different clinicobiological subtypes (the Kaplan–Meier curve on the right shows chronic lymphocytic leukemia [CLL] as an example). Cell proliferation is also imprinted into the DNA methylome, which allows to determine the past proliferative history (epigenetically determined cumulative mitoses score), whose magnitude also has important prognostic value in B‐cell malignancies (lower panel). (b) Histone modifications, chromatin accessibility, and 3D genome interactions are important epigenetic marks (left). The combination of different histone marks allows identification of segments of the genome with different functions, the so‐called chromatin states (right). (c) Example of multilayered epigenetic analysis for SOX11 in mantle cell lymphoma (MCL). Chromatin state analysis in SOX11 positive MCL shows active promoter/distal super‐enhancer regions, which are in close contact in the 3D space (chromatin loop). SOX11 negative MCL has a poised promoter and a small weak enhancer, which are maintained distant in the 3D space (no chromatin loop).
