Abstract
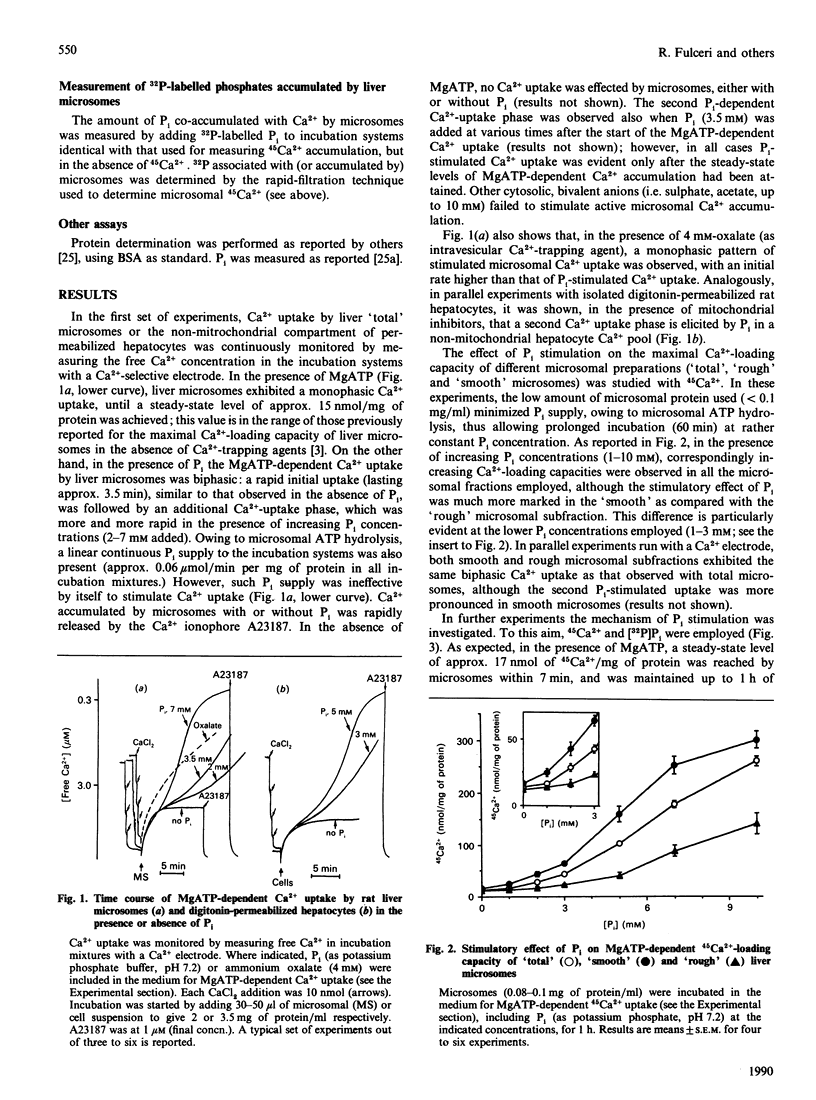
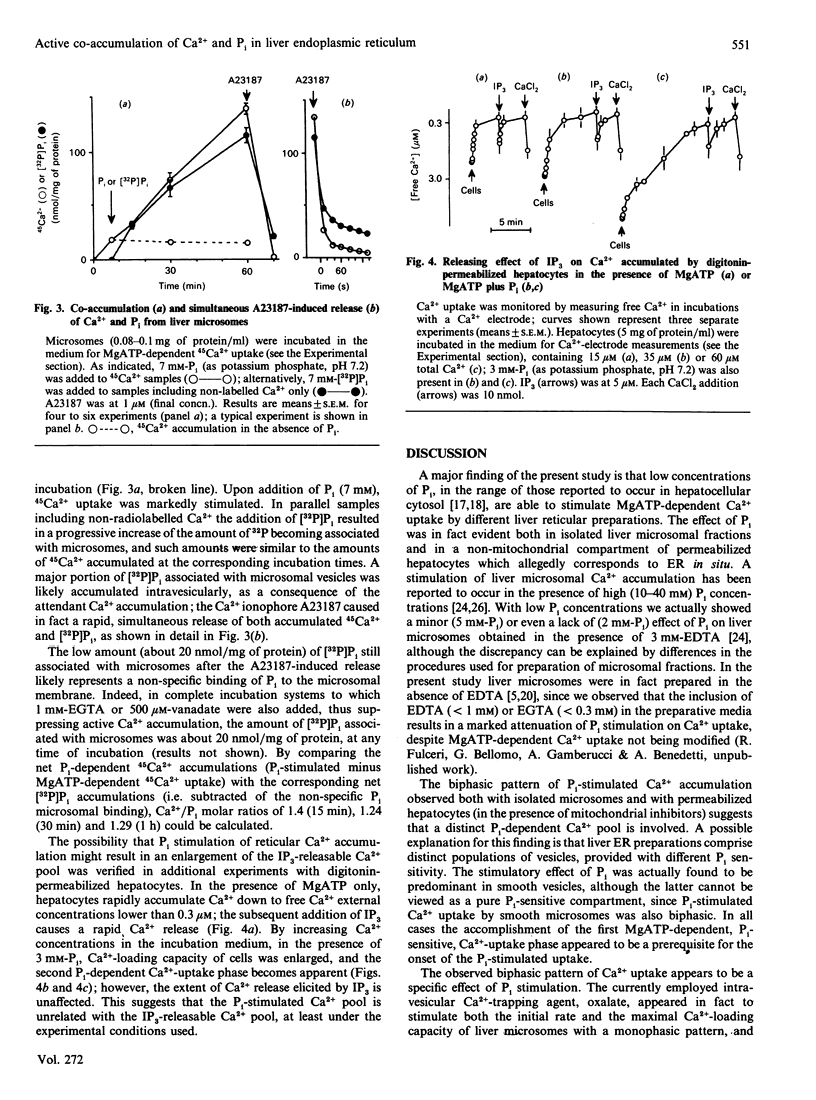
1. MgATP-dependent Ca2+ uptake by rat liver microsomal preparations and permeabilized hepatocytes was measured in the presence or absence of Pi. 2. Monitoring of free Ca2+ in incubation systems with a Ca2+ electrode in the presence of Pi (2-7 mM) revealed a biphasic Ca2+ uptake, with the onset of a second, Pi-dependent, Ca2+ accumulation. 3. Increasing Pi concentrations (up to 10 mM) caused a progressive enlargement of 45Ca2(+)-loading capacity of microsomal fractions. 4. As a result of Pi stimulation of active Ca2+ uptake, [32P]Pi and 45Ca2+ were co-accumulated. 5. Experiments with permeabilized hepatocytes revealed that the amount of Ca2+ releasable by myo-inositol 1,4,5-trisphosphate is unaffected by Pi.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affolter H., Sigel E. A simple system for the measurement of ion activities with solvent polymeric membrane electrodes. Anal Biochem. 1979 Sep 1;97(2):315–319. doi: 10.1016/0003-2697(79)90078-2. [DOI] [PubMed] [Google Scholar]
- Akerboom T. P., Bookelman H., Zuurendonk P. F., van der Meer R., Tager J. M. Intramitochondrial and extramitochondrial concentrations of adenine nucleotides and inorganic phosphate in isolated hepatocytes from fasted rats. Eur J Biochem. 1978 Mar 15;84(2):413–420. doi: 10.1111/j.1432-1033.1978.tb12182.x. [DOI] [PubMed] [Google Scholar]
- Benedetti A., Fulceri R., Comporti M. Calcium sequestration activity in rat liver microsomes. Evidence for a cooperation of calcium transport with glucose-6-phosphatase. Biochim Biophys Acta. 1985 Jun 27;816(2):267–277. doi: 10.1016/0005-2736(85)90494-8. [DOI] [PubMed] [Google Scholar]
- Benedetti A., Fulceri R., Romani A., Comporti M. MgATP-dependent glucose 6-phosphate-stimulated Ca2+ accumulation in liver microsomal fractions. Effects of inositol 1,4,5-trisphosphate and GTP. J Biol Chem. 1988 Mar 5;263(7):3466–3473. [PubMed] [Google Scholar]
- Berridge M. J. Inositol lipids and cell proliferation. Biochim Biophys Acta. 1987 Apr 20;907(1):33–45. doi: 10.1016/0304-419x(87)90017-5. [DOI] [PubMed] [Google Scholar]
- Brattin W. J., Jr, Waller R. L., Recknagel R. O. Analysis of microsomal calcium sequestration by steady state isotope exchange. Enzyme kinetics and role of membrane permeability. J Biol Chem. 1982 Sep 10;257(17):10044–10051. [PubMed] [Google Scholar]
- Bygrave F. L., Anderson T. A. Ruthenium red-insensitive calcium transport in ascites-sarcoma 180/TG cells. Biochem J. 1981 Nov 15;200(2):343–348. doi: 10.1042/bj2000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bygrave F. L., Lenton L., Altin J. G., Setchell B. A., Karjalainen A. Phosphate and calcium uptake by mitochondria and by perfused rat liver induced by the synergistic action of glucagon and vasopressin. Biochem J. 1990 Apr 1;267(1):69–73. doi: 10.1042/bj2670069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bygrave F. L. Mitochondria and the control of intracellular calcium. Biol Rev Camb Philos Soc. 1978 Feb;53(1):43–79. doi: 10.1111/j.1469-185x.1978.tb00992.x. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Cerdan S., Seelig J. NMR studies of metabolism. Annu Rev Biophys Biophys Chem. 1990;19:43–67. doi: 10.1146/annurev.bb.19.060190.000355. [DOI] [PubMed] [Google Scholar]
- Chan K. M., Koepnick S. L. The mechanism of calcium uptake by liver microsomes: effect of anions and ionophores. Biochim Biophys Acta. 1985 Sep 10;818(3):291–298. doi: 10.1016/0005-2736(85)90002-1. [DOI] [PubMed] [Google Scholar]
- Dallner G. Isolation of microsomal subfractions by use of density gradients. Methods Enzymol. 1978;52:71–82. doi: 10.1016/s0076-6879(78)52007-7. [DOI] [PubMed] [Google Scholar]
- Dean W. L. Purification and reconstitution of a Ca2+ pump from human platelets. J Biol Chem. 1984 Jun 10;259(11):7343–7348. [PubMed] [Google Scholar]
- Feher J. J., Lipford G. B. Calcium oxalate and calcium phosphate capacities of cardiac sarcoplasmic reticulum. Biochim Biophys Acta. 1985 Sep 10;818(3):373–385. doi: 10.1016/0005-2736(85)90012-4. [DOI] [PubMed] [Google Scholar]
- Henne V., Söling H. D. Guanosine 5'-triphosphate releases calcium from rat liver and guinea pig parotid gland endoplasmic reticulum independently of inositol 1,4,5-trisphosphate. FEBS Lett. 1986 Jul 7;202(2):267–273. doi: 10.1016/0014-5793(86)80699-8. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Williamson J. R. Inositol polyphosphates and intracellular calcium release. Arch Biochem Biophys. 1989 Aug 15;273(1):1–15. doi: 10.1016/0003-9861(89)90156-2. [DOI] [PubMed] [Google Scholar]
- Käser-Glanzmann R., Jakábová M., George J. N., Lüscher E. F. Further characterization of calcium-accumulating vesicles from human blood platelets. Biochim Biophys Acta. 1978 Sep 11;512(1):1–12. doi: 10.1016/0005-2736(78)90213-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979 Nov 15;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L., Carafoli E., Rossi C. S. Energy-linked ion movements in mitochondrial systems. Adv Enzymol Relat Areas Mol Biol. 1967;29:259–320. doi: 10.1002/9780470122747.ch6. [DOI] [PubMed] [Google Scholar]
- Moldéus P., Högberg J., Orrenius S. Isolation and use of liver cells. Methods Enzymol. 1978;52:60–71. doi: 10.1016/s0076-6879(78)52006-5. [DOI] [PubMed] [Google Scholar]
- Muallem S., Schoeffield M., Pandol S., Sachs G. Inositol trisphosphate modification of ion transport in rough endoplasmic reticulum. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4433–4437. doi: 10.1073/pnas.82.13.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke F. A., Halenda S. P., Zavoico G. B., Feinstein M. B. Inositol 1,4,5-trisphosphate releases Ca2+ from a Ca2+-transporting membrane vesicle fraction derived from human platelets. J Biol Chem. 1985 Jan 25;260(2):956–962. [PubMed] [Google Scholar]
- Romani A., Fulceri R., Pompella A., Ferro M., Benedetti A. Active Ca2+ accumulation in the endoplasmic reticulum of different hepatomas: stimulation by phosphates and Ca2+-releasing effect of IP3. Ann N Y Acad Sci. 1988;551:249–252. doi: 10.1111/j.1749-6632.1988.tb22343.x. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Bond M., Somlyo A. V. Calcium content of mitochondria and endoplasmic reticulum in liver frozen rapidly in vivo. Nature. 1985 Apr 18;314(6012):622–625. doi: 10.1038/314622a0. [DOI] [PubMed] [Google Scholar]
- Trotta E. E., de Meis L. ATP-dependent calcium accumulation in brain microsomes. Enhancement by phosphate and oxalate. Biochim Biophys Acta. 1975 Jun 25;394(2):239–247. doi: 10.1016/0005-2736(75)90262-x. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Ca2+-selective electrodes: a novel PVC-gelled neutral carrier mixture compared with other currently available sensors. J Neurosci Methods. 1981 Jun;4(1):73–86. doi: 10.1016/0165-0270(81)90020-0. [DOI] [PubMed] [Google Scholar]
- Volpe P., Krause K. H., Hashimoto S., Zorzato F., Pozzan T., Meldolesi J., Lew D. P. "Calciosome," a cytoplasmic organelle: the inositol 1,4,5-trisphosphate-sensitive Ca2+ store of nonmuscle cells? Proc Natl Acad Sci U S A. 1988 Feb;85(4):1091–1095. doi: 10.1073/pnas.85.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell I. D., Lindsay J. G., Burchell A. The identification of T2; the phosphate/pyrophosphate transport protein of the hepatic microsomal glucose-6-phosphatase system. FEBS Lett. 1988 Feb 29;229(1):179–182. doi: 10.1016/0014-5793(88)80822-6. [DOI] [PubMed] [Google Scholar]


