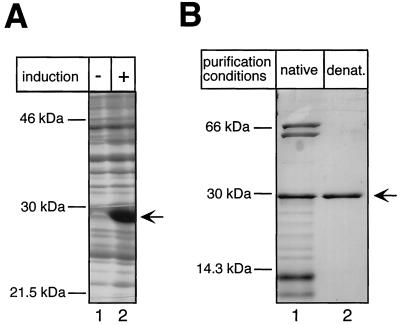
FIG. 4.
Bacterial expression and purification of the DHBV TP domain. (A) Expression of TP1-220 in E. coli BL21-CodonPlus-RIL cells. E. coli cultures transformed with plasmid pET-TP1-220 were grown in the presence (+) or absence (−) of IPTG, and the pelleted cells were lysed by boiling in SDS sample buffer. Total lysates were analyzed by SDS-PAGE and Coomassie blue staining. (B) Purification of TP1-220. The soluble fraction of TP1-220 was subjected to IMAC and subsequent dialysis against a physiological buffer. The insoluble faction was solubilized in 8 M urea and purified by IMAC under denaturing (denat.) conditions. The protein was refolded by two-step dialysis against buffers containing 3 and 1 M urea, respectively. Aliquots of both purified fractions were resolved by SDS-PAGE and stained with Coomassie blue. Note the presence in the natively purified fraction (lane 1) of additional proteins of about 70 , 60, and <14 kDa. The former two correspond to E. coli DnaK and GroEL, as indicated by Western blotting with specific antibodies (not shown).