Abstract
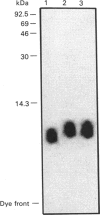
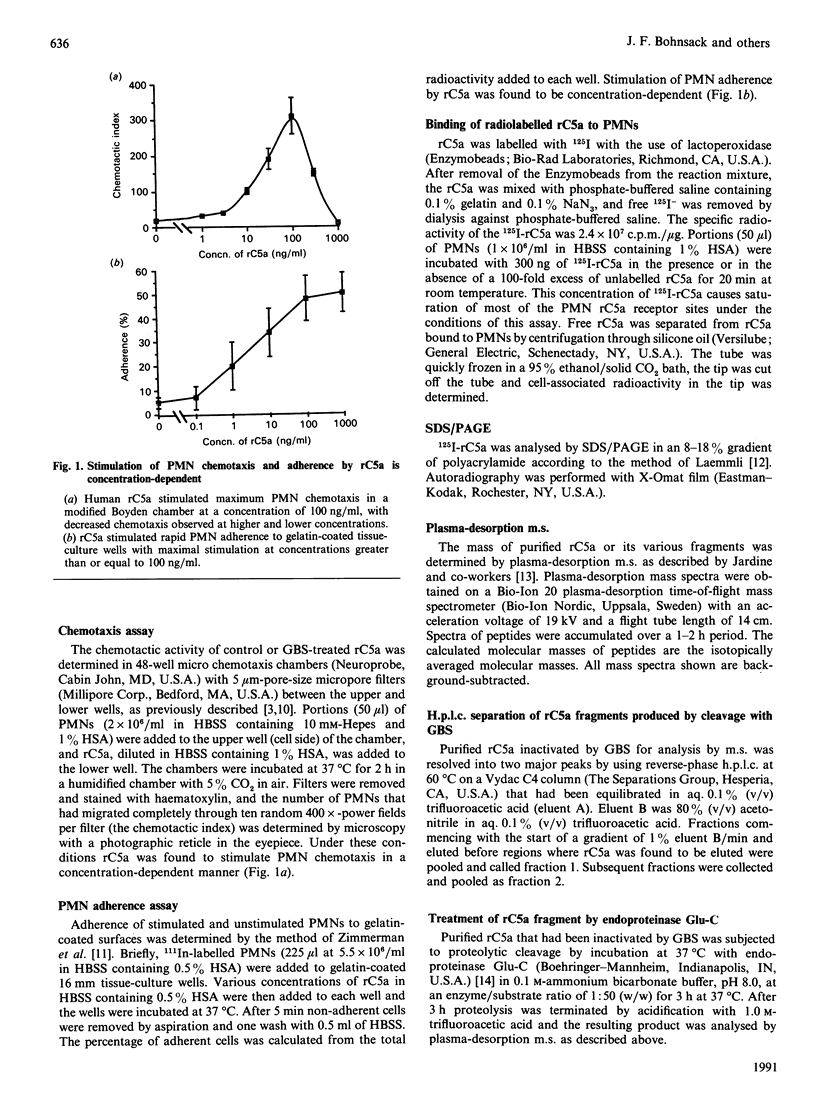
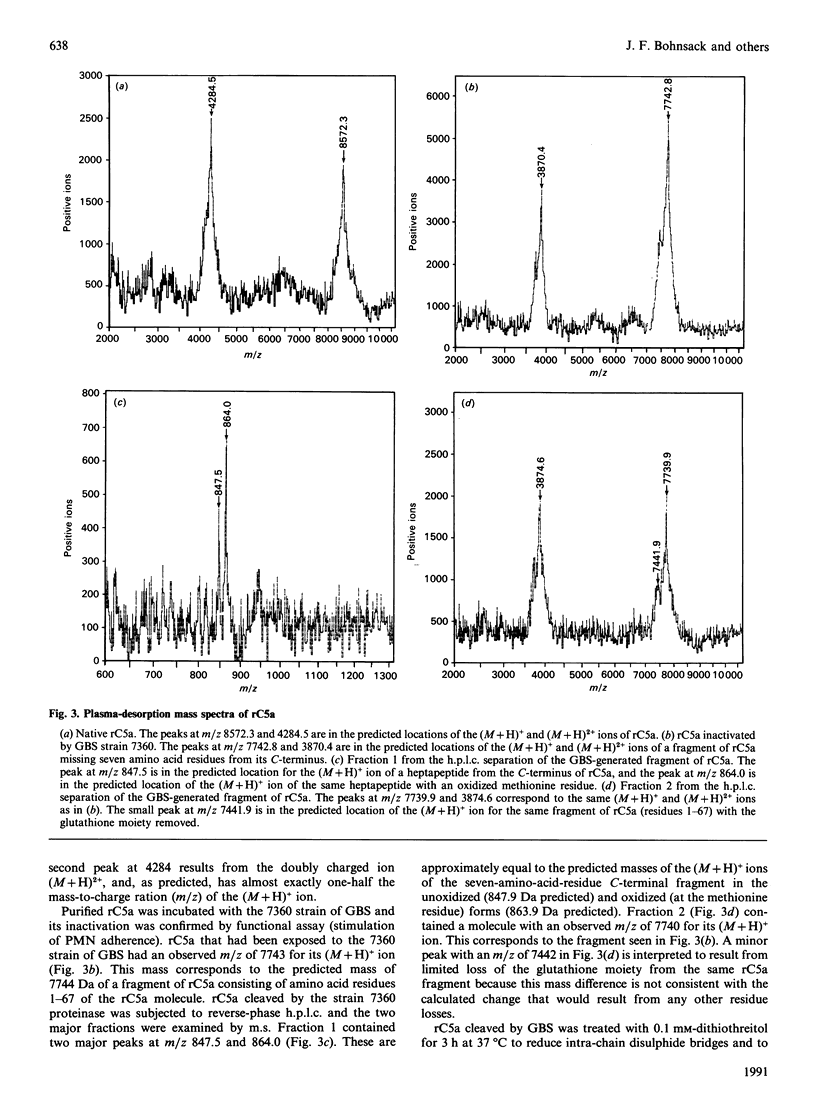
Incubation of recombinant human C5a (rC5a) with the 7360 strain of group B streptococci (GBS) destroyed the ability of rC5a to stimulate chemotaxis or adherence of purified human polymorphonuclear leucocytes (PMNs). Treatment of 125I-labelled rC5a with GBS 7360 correspondingly decreased rC5a binding to human PMNs. This also resulted in an approx. 600 Da decrease in the molecular mass of rC5a as determined by SDS/PAGE. Incubation of rC5a with the GBS strain GW, which only minimally altered the ability of rC5a to activate human PMNs, did not affect rC5a binding to PMNs and did not alter the molecular mass of rC5a on SDS/PAGE. Plasma-desorption m.s. of rC5a inactivated by GBS 7360 showed that the GBS cleaved the rC5a between histidine-67 and lysine-68 near the C-terminus of rC5a. This mechanism of inactivation of C5a by proteolytic cleavage at the C-terminus of C5a is consistent with the known critical role of the C-terminus in C5a activation of human PMNs. This C5a-cleaving proteinase activity may contribute to the pathophysiology of GBS infections.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony B. F., Okada D. M. The emergence of group B streptococci in infections of the newborn infant. Annu Rev Med. 1977;28:355–369. doi: 10.1146/annurev.me.28.020177.002035. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Kasper D. L. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med. 1976 Apr 1;294(14):753–756. doi: 10.1056/NEJM197604012941404. [DOI] [PubMed] [Google Scholar]
- Cotter R. J. Plasma desorption mass spectrometry: coming of age. Anal Chem. 1988 Jul 1;60(13):781A–793A. doi: 10.1021/ac00164a002. [DOI] [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Gerard C., Chenoweth D. E., Hugli T. E. Molecular aspects of the serum chemotactic factors. J Reticuloendothel Soc. 1979 Dec;26(Suppl):711–718. [PubMed] [Google Scholar]
- Gerard C., Hugli T. E. Identification of classical anaphylatoxin as the des-Arg form of the C5a molecule: evidence of a modulator role for the oligosaccharide unit in human des-Arg74-C5a. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1833–1837. doi: 10.1073/pnas.78.3.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper T. E., Christensen R. D., Rothstein G., Hill H. R. Effect of intravenous immunoglobulin G on neutrophil kinetics during experimental group B streptococcal infection in neonatal rats. Rev Infect Dis. 1986 Jul-Aug;8 (Suppl 4):S401–S408. doi: 10.1093/clinids/8.supplement_4.s401. [DOI] [PubMed] [Google Scholar]
- Hemming V. G., Hall R. T., Rhodes P. G., Shigeoka A. O., Hill H. R. Assessment of group B streptococcal opsonins in human and rabbit serum by neutrophil chemiluminescence. J Clin Invest. 1976 Dec;58(6):1379–1387. doi: 10.1172/JCI108593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming V. G., McCloskey D. W., Hill H. R. Pneumonia in the neonate associated with group B streptococcal septicemia. Am J Dis Child. 1976 Nov;130(11):1231–1233. doi: 10.1001/archpedi.1976.02120120065011. [DOI] [PubMed] [Google Scholar]
- Hill H. R., Augustine N. H., Newton J. A., Shigeoka A. O., Morris E., Sacchi F. Correction of a developmental defect in neutrophil activation and movement. Am J Pathol. 1987 Aug;128(2):307–314. [PMC free article] [PubMed] [Google Scholar]
- Hill H. R., Bohnsack J. F., Morris E. Z., Augustine N. H., Parker C. J., Cleary P. P., Wu J. T. Group B streptococci inhibit the chemotactic activity of the fifth component of complement. J Immunol. 1988 Nov 15;141(10):3551–3556. [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugli T. E. Structure and function of the anaphylatoxins. Springer Semin Immunopathol. 1984;7(2-3):193–219. doi: 10.1007/BF01893020. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandecki W., Mollison K. W., Bolling T. J., Powell B. S., Carter G. W., Fox J. L. Chemical synthesis of a gene encoding the human complement fragment C5a and its expression in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3543–3547. doi: 10.1073/pnas.82.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandecki W., Powell B. S., Mollison K. W., Carter G. W., Fox J. L. High-level expression of a gene encoding the human complement factor C5a in Escherichia coli. Gene. 1986;43(1-2):131–138. doi: 10.1016/0378-1119(86)90016-8. [DOI] [PubMed] [Google Scholar]
- Mollison K. W., Fey T. A., Krause R. A., Mandecki W., Fox J. L., Carter G. W. High-level C5a gene expression and recovery of recombinant human C5a from Escherichia coli. Agents Actions. 1987 Aug;21(3-4):366–370. doi: 10.1007/BF01966518. [DOI] [PubMed] [Google Scholar]
- Mollison K. W., Mandecki W., Zuiderweg E. R., Fayer L., Fey T. A., Krause R. A., Conway R. G., Miller L., Edalji R. P., Shallcross M. A. Identification of receptor-binding residues in the inflammatory complement protein C5a by site-directed mutagenesis. Proc Natl Acad Sci U S A. 1989 Jan;86(1):292–296. doi: 10.1073/pnas.86.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsarbopoulos A., Becker G. W., Occolowitz J. L., Jardine I. Peptide and protein mapping by 252Cf-plasma desorption mass spectrometry. Anal Biochem. 1988 May 15;171(1):113–123. doi: 10.1016/0003-2697(88)90131-5. [DOI] [PubMed] [Google Scholar]
- Wexler D. E., Chenoweth D. E., Cleary P. P. Mechanism of action of the group A streptococcal C5a inactivator. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8144–8148. doi: 10.1073/pnas.82.23.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman G. A., McIntyre T. M., Prescott S. M. Thrombin stimulates neutrophil adherence by an endothelial cell-dependent mechanism: characterization of the response and relationship to platelet-activating factor synthesis. Ann N Y Acad Sci. 1986;485:349–368. doi: 10.1111/j.1749-6632.1986.tb34596.x. [DOI] [PubMed] [Google Scholar]