Fig. 1.
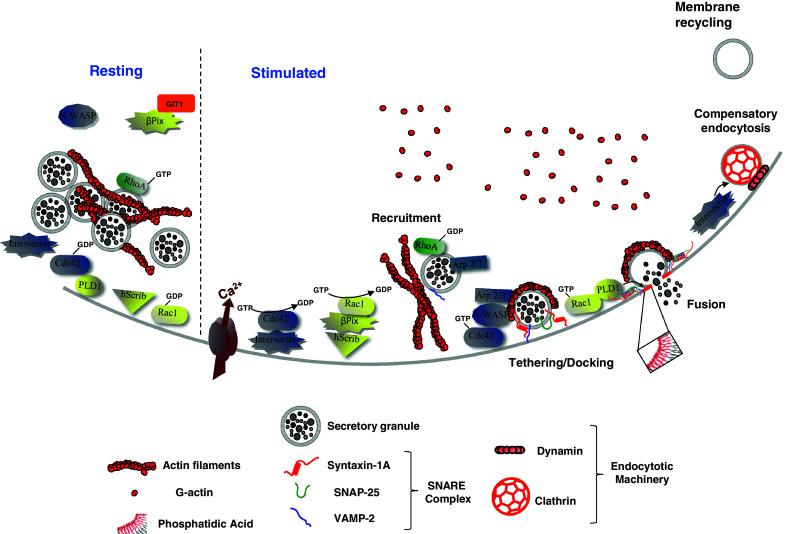
Model of the hypothetical roles of Rho GTPases, Intersectin-1L and β-Pix in calcium-regulated exocytosis from neuroendocrine chromaffin cells. In resting chromaffin cells, secretory granule-associated RhoA is activated and contributes to the stabilization of the cortical actin network. Secretagogue stimulation triggers the recruitment and docking of secretory granules to the plasma membrane. In parallel, while RhoA is presumably inactivated to facilitate actin reorganisation, intersectin-1L activates Cdc42 at exocytotic sites, whereas hScrib recruits β-Pix at the plasma membrane, which in turn activates Rac1. At a post-docking stage, activated-Cdc42 triggers the formation of actin filaments through N-WASP and the granule-bound Arp 2/3 complex. These de novo actin structures, localized at exocytotic sites, are required for some late stages of exocytosis. GTP-bound Rac1 stimulates phospholipase D1 to produce membrane-localized phosphatidic acid at the granule docking site to facilitate formation of the fusion pore. With a well documented function in endocytosis, Intersectin-1L may couple the Cdc42-dependent actin remodelling necessary for exocytosis and the recruitment of the endocytotic machinery required for the subsequent granule membrane retrieval and recycling. When cells return to a resting state, Rac1 and Cdc42 are inactivated through currently unidentified GTPase-activating proteins
