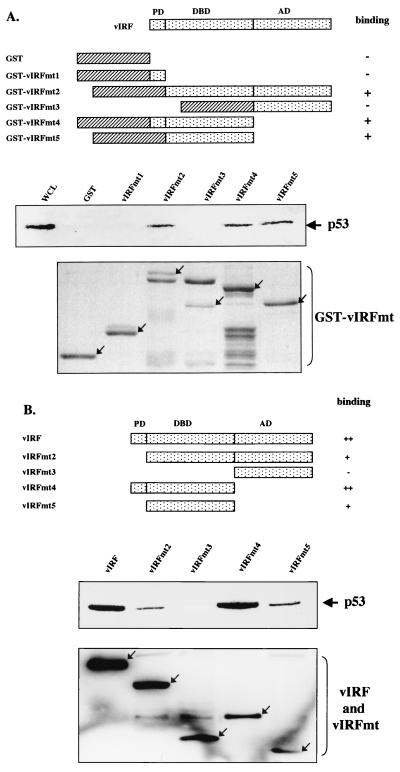
FIG. 3.
Mutational analysis of vIRF for p53 interaction. (A) Summary of GST-vIRF fusion constructs and in vitro GST pull-down assay. Individual domains of vIRF were cloned into the pGEX4T-1 vector to generate GST-vIRF fusion proteins. Lysates of Saos-2 cells infected with Ad-p53 were precleared with 5 μg of GST, followed by incubation with 5 μg of GST or GST-vIRF fusion proteins. Polypeptides associated with the GST-vIRF fusion proteins were subject to Western blotting with an anti-p53 antibody. A whole-cell lysate (WCL) which represents 5% of cellular p53 was used for a positive control. Arrows in the bottom indicate GST and GST-vIRF mutant fusion proteins (GST-vIRFmt) stained with Coomassie blue solution. Boxes with slashed lines indicate GST, and boxes with dots indicate a domain of vIRF, PD, proline-rich domain; DBD, DNA binding domain; AD, activation domain. + and − indicate positive and negative binding of GST-vIRF fusion proteins to p53. (B) Summary of vIRF mutants and in vivo interaction of vIRF mutants with p53. COS-1 cells were transfected with the Flag-tagged wt vIRF and mutants vIRFmt2 to -5. Cell lysates were used for immunoprecipitation with an anti-Flag antibody, followed by Western blotting with an anti-p53 antibody to detect p53. PD, proline-rich domain; DBD, DNA binding domain; AD, activation domain. Western blotting of whole-cell lysates with an anti-Flag antibody showed equivalent expression of wt and mutant vIRF: arrows indicate the Flag-tagged wt and mutant vIRF (bottom). +, ++, and − indicate weak, strong, and no binding of vIRF mutants to p53.