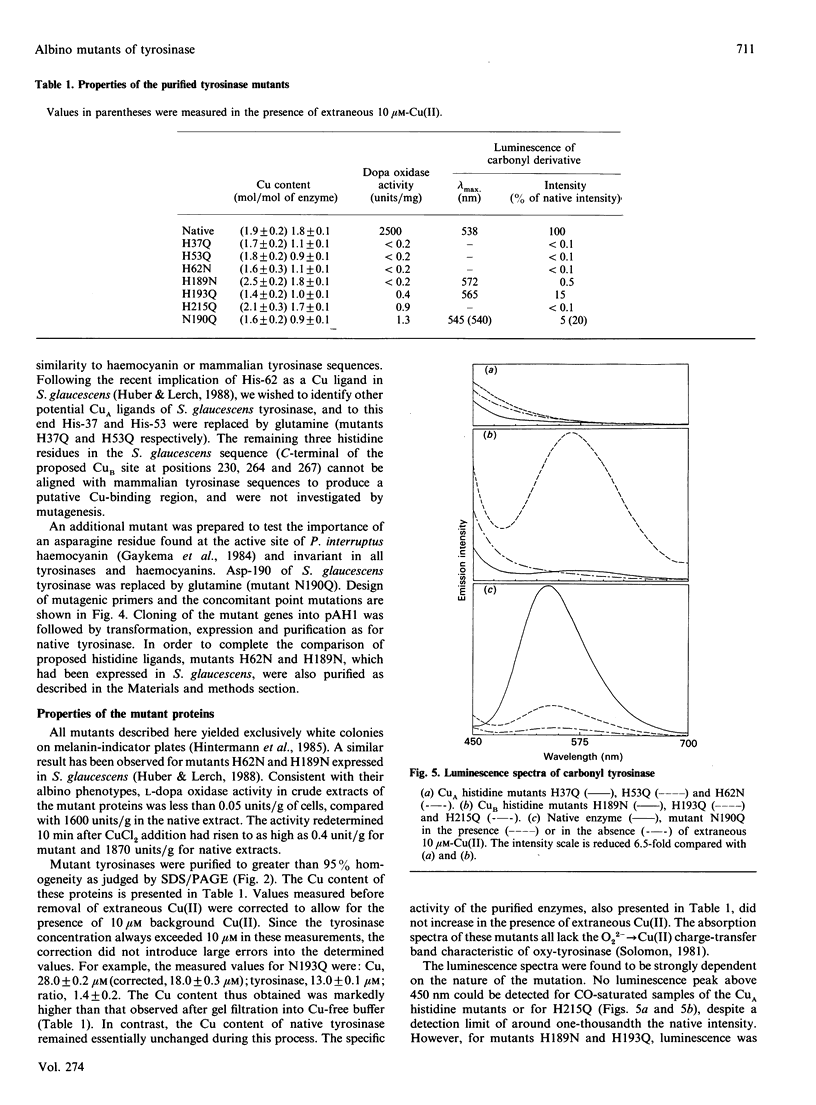
Abstract
Site-directed mutagenesis was used to determine the functional role of several residues of Streptomyces glaucescens tyrosinase. Replacement of His-37, -53, -193 or -215 by glutamine yields albino phenotypes, as determined by expression on melanin-indicator plates. The purified mutant proteins display no detectable oxy-enzyme and increased Cu lability at the binuclear active site. The carbonyl derivatives of H189Q and H193Q luminesce, with lambda max. displaced more than 25 nm to a longer wavelength compared with native tyrosinase. The remaining histidine mutants display no detectable luminescence. The results are consistent with these histidine residues (together with His-62 and His-189 reported earlier) acting as Cu ligands in the Streptomyces glaucescens enzyme. Conservative substitution of the invariant Asn-190 by glutamine also gives an albino phenotype, no detectable oxy-enzyme and labilization of active-site Cu. The luminescence spectrum of carbonyl-N190Q, however, closely resembles that of the native enzyme under conditions promoting double Cu occupancy of the catalytic site. A critical role for Asn-190 in active-site hydrogen-bonding interactions is proposed.
Full text
PDF
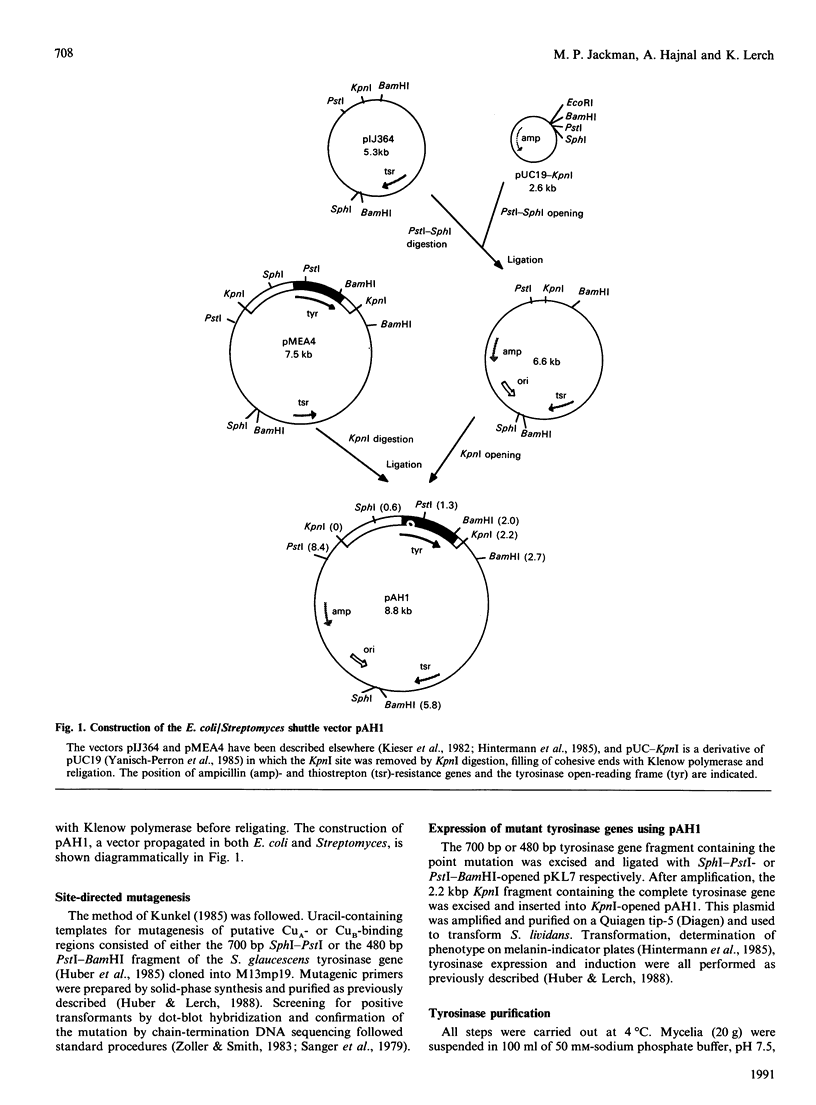
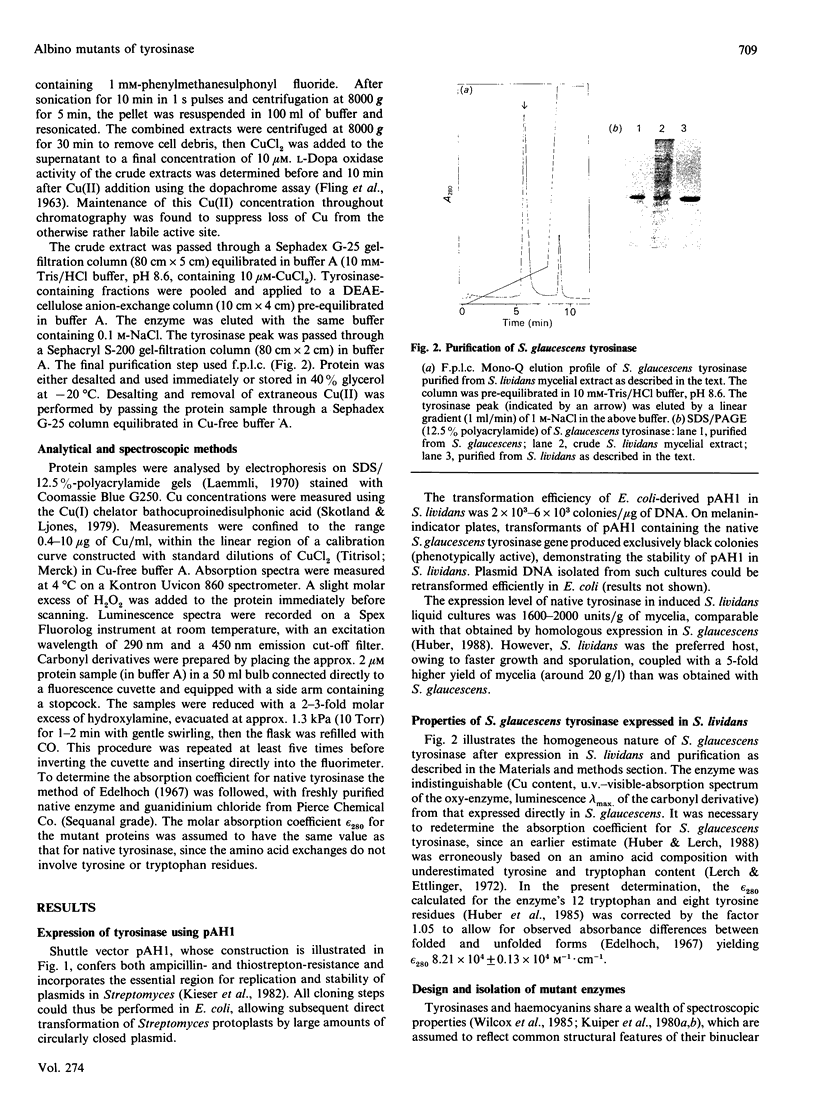
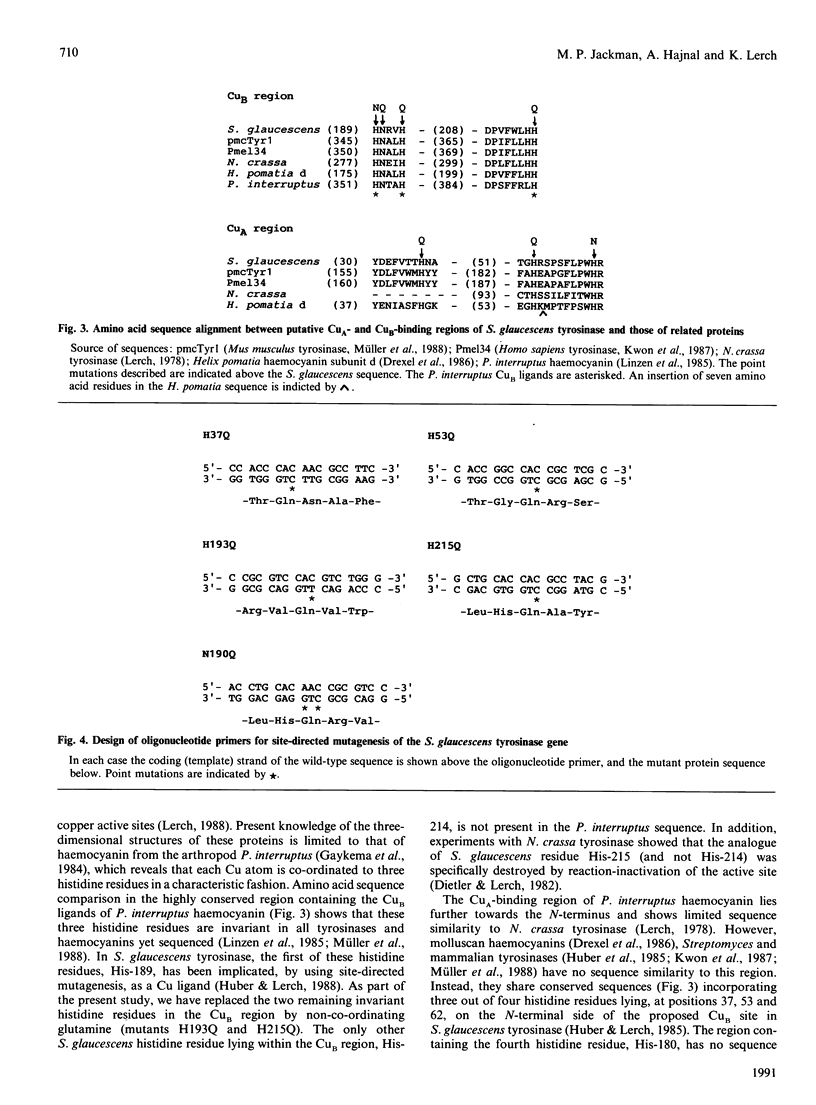


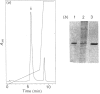
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- FLING M., HOROWITZ N. H., HEINEMANN S. F. The isolation and properties of crystalline tyrosinase from Neurospora. J Biol Chem. 1963 Jun;238:2045–2053. [PubMed] [Google Scholar]
- Fager L. Y., Alben J. O. Structure of the carbon monoxide binding site of hemocyanins studied by Fourier transform infrared spectroscopy. Biochemistry. 1972 Dec 5;11(25):4786–4792. doi: 10.1021/bi00775a023. [DOI] [PubMed] [Google Scholar]
- Finazzi-Agrò A., Zolla L., Flamigni L., Kuiper H. A., Brunori M. Spectroscopy of (carbon monoxy)hemocyanins. Phosphorescence of the binuclear carbonylated copper centers. Biochemistry. 1982 Jan 19;21(2):415–418. doi: 10.1021/bi00531a032. [DOI] [PubMed] [Google Scholar]
- Hintermann G., Zatchej M., Hütter R. Cloning and expression of the genetically unstable tyrosinase structural gene from Streptomyces glaucescens. Mol Gen Genet. 1985;200(3):422–432. doi: 10.1007/BF00425726. [DOI] [PubMed] [Google Scholar]
- Huber M., Hintermann G., Lerch K. Primary structure of tyrosinase from Streptomyces glaucescens. Biochemistry. 1985 Oct 22;24(22):6038–6044. doi: 10.1021/bi00343a003. [DOI] [PubMed] [Google Scholar]
- Huber M., Lerch K. Identification of two histidines as copper ligands in Streptomyces glaucescens tyrosinase. Biochemistry. 1988 Jul 26;27(15):5610–5615. doi: 10.1021/bi00415a032. [DOI] [PubMed] [Google Scholar]
- Kieser T., Hopwood D. A., Wright H. M., Thompson C. J. pIJ101, a multi-copy broad host-range Streptomyces plasmid: functional analysis and development of DNA cloning vectors. Mol Gen Genet. 1982;185(2):223–228. doi: 10.1007/BF00330791. [DOI] [PubMed] [Google Scholar]
- Knowles J. R. Tinkering with enzymes: what are we learning? Science. 1987 Jun 5;236(4806):1252–1258. doi: 10.1126/science.3296192. [DOI] [PubMed] [Google Scholar]
- Kuiper H. A., Agrò A. F., Antonini E., Brunori M. Luminescence of carbon monoxide hemocyanins. Proc Natl Acad Sci U S A. 1980 May;77(5):2387–2389. doi: 10.1073/pnas.77.5.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper H. A., Lerch K., Brunori M., Finazzi Agrò A. Luminescence of the copper--carbon monoxide complex of Neurospora tyrosinase. FEBS Lett. 1980 Feb 25;111(1):232–234. doi: 10.1016/0014-5793(80)80800-3. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon B. S., Haq A. K., Pomerantz S. H., Halaban R. Isolation and sequence of a cDNA clone for human tyrosinase that maps at the mouse c-albino locus. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7473–7477. doi: 10.1073/pnas.84.21.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerch K. Amino acid sequence of tyrosinase from Neurospora crassa. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3635–3639. doi: 10.1073/pnas.75.8.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch K., Ettinger L. Purification and characterization of a tyrosinase from Streptomyces glaucescens. Eur J Biochem. 1972 Dec 18;31(3):427–437. doi: 10.1111/j.1432-1033.1972.tb02549.x. [DOI] [PubMed] [Google Scholar]
- Lerch K. Neurospora tyrosinase: structural, spectroscopic and catalytic properties. Mol Cell Biochem. 1983;52(2):125–138. doi: 10.1007/BF00224921. [DOI] [PubMed] [Google Scholar]
- Linzen B., Soeter N. M., Riggs A. F., Schneider H. J., Schartau W., Moore M. D., Yokota E., Behrens P. Q., Nakashima H., Takagi T. The structure of arthropod hemocyanins. Science. 1985 Aug 9;229(4713):519–524. doi: 10.1126/science.4023698. [DOI] [PubMed] [Google Scholar]
- MASON H. S. OXIDASES. Annu Rev Biochem. 1965;34:595–634. doi: 10.1146/annurev.bi.34.070165.003115. [DOI] [PubMed] [Google Scholar]
- Müller G., Ruppert S., Schmid E., Schütz G. Functional analysis of alternatively spliced tyrosinase gene transcripts. EMBO J. 1988 Sep;7(9):2723–2730. doi: 10.1002/j.1460-2075.1988.tb03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skotland T., Ljones T. The enzyme-bound copper of dopamine beta-monooxygenase. Reaction with copper chelators, preparation of the apoprotein, and kinetics of the reconstitution by added copper. Eur J Biochem. 1979 Feb 15;94(1):145–151. doi: 10.1111/j.1432-1033.1979.tb12881.x. [DOI] [PubMed] [Google Scholar]
- Sorrell T. N., Beltramini M., Lerch K. Luminescence of deoxyhemocyanin and deoxytyrosinase. J Biol Chem. 1988 Jul 15;263(20):9576–9577. [PubMed] [Google Scholar]
- Uiterkamp A. J., Mason H. S. Magnetic dipole-dipole coupled Cu(II) pairs in nitric oxide-treated tyrosinase: a structural relationship between the active sites of tyrosinase and hemocyanin. Proc Natl Acad Sci U S A. 1973 Apr;70(4):993–996. doi: 10.1073/pnas.70.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]