Abstract
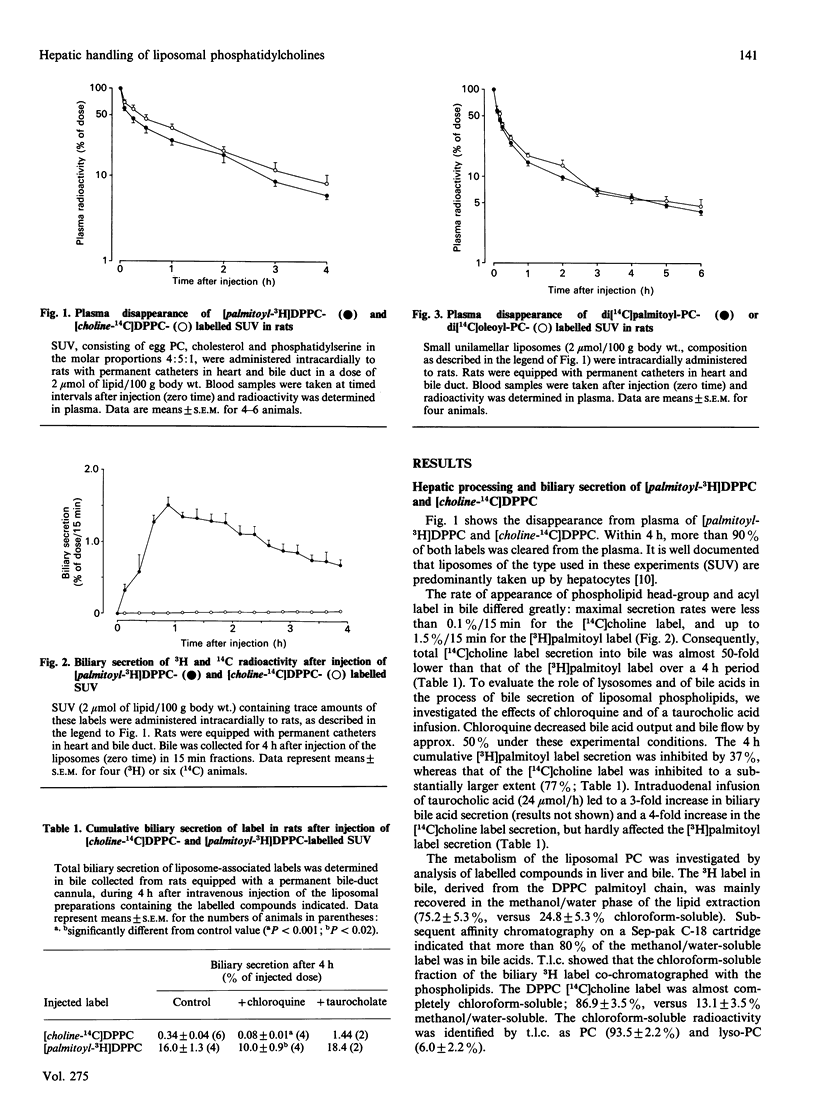
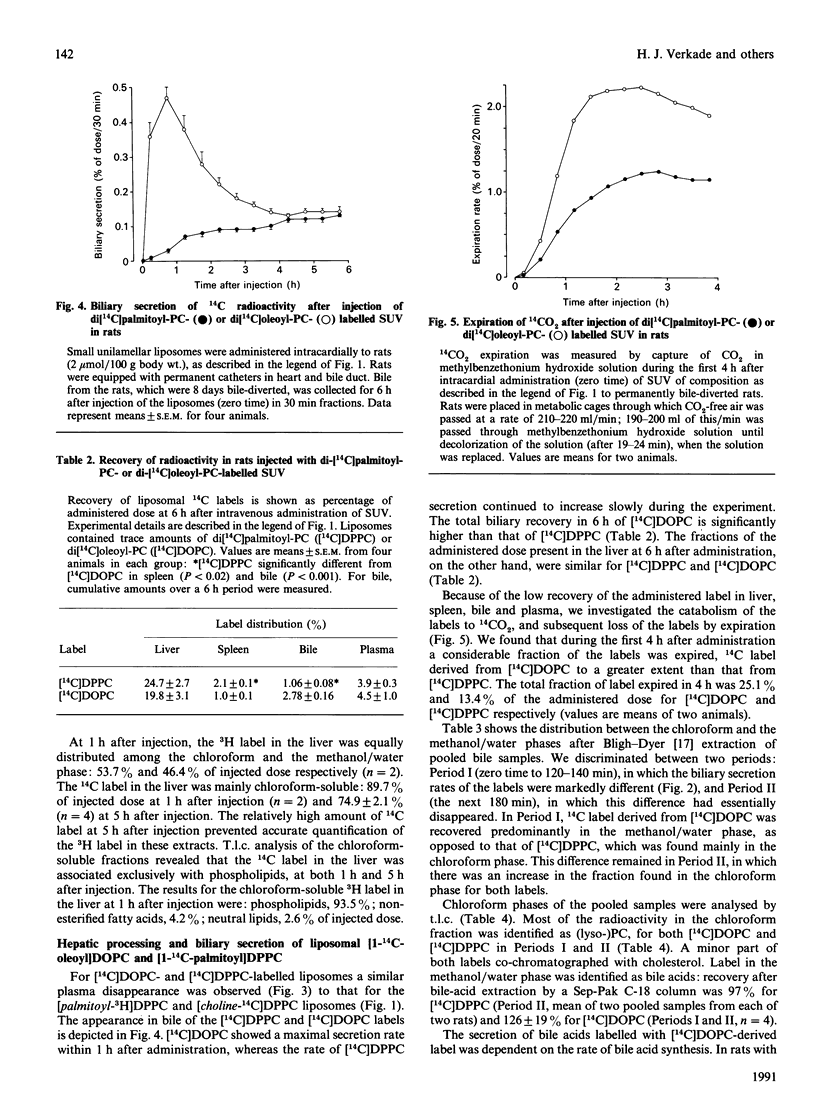
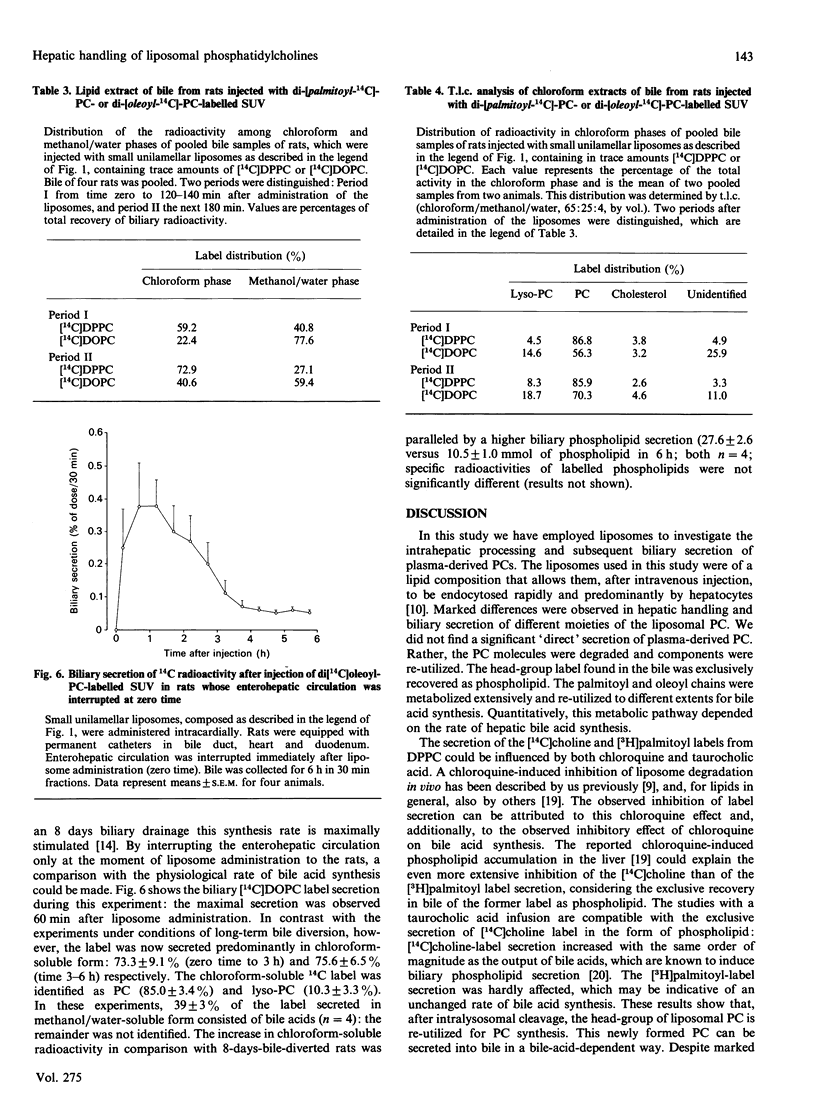
To investigate the contribution of plasma-derived phosphatidylcholine (PC) to bile PC, the hepatic processing and biliary secretion of liposome-associated PC was studied in rats. For this purpose, small unilamellar vesicles (SUV), containing trace amounts of [2-palmitoyl-9,10-3H]dipalmitoylphosphatidylcholine ([palmitoyl-3H]DPPC), [choline-14C]-dipalmitoylphosphatidylcholine ([choline-14C]DPPC), di[14C]palmitoylphosphatidylcholine ([14C]DPPC) or di[1-14C]-oleoylphosphatidylcholine ([14C]DOPC), were administered intravenously to unanaesthetized rats, equipped with permanent catheters in heart and bile duct. Biliary secretion of the 14C-head-group label of DPPC was very slow (0.3% of injected dose in 4 h), whereas the [3H]palmitoyl label was secreted at a much higher rate (16% in 4 h), but only after substantial catabolism of the acyl chain. To study the latter process in more detail, we compared hepatic metabolism and biliary secretion of [1-14C]acyl-labelled DPPC and DOPC. In rats with an 8-day bile drainage, degradation products of the oleoyl chain were utilized for synthesis of bile acids, which were subsequently secreted into the bile (2% in 6 h). A much smaller fraction (0.6% in 6 h) was secreted as PC and lyso-PC. When bile drainage was started immediately after SUV injection, i.e. a situation with a low hepatic bile acid synthesis rate and a high phospholipid secretion, the secretion of [14C]DOPC-derived radioactivity in the form of bile acids was decreased (0.2% in 6 h), and that as (lyso-)PC increased (1.5% in 6 h). Biliary secretion of DPPC palmitoyl chains in bile-diverted rats was much less than that of the oleoyl chains, and occurred predominantly as PC and lyso-PC (0.6%, compared with 0.4% as bile acids in 6 h). Breath analyses demonstrated that a considerable fraction of both acyl chains was oxidized to CO2 and expired: 25.1% of the administered label for oleoyl chains and 13.4% for palmitoyl chains respectively in a 4 h period. The results of this study indicate that liposomal PC is only minimally secreted into bile via a direct pathway; the bulk is extensively degraded in the liver. Resulting products are partly secreted into bile, as bile acid or as resynthesized PC. There appears to be a quantitative difference in the metabolism of oleoyl and palmitoyl acyl chains.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvaro D., Angelico M., Cantafora A., Di Biase A., De Santis A., Bracci F., Minervini G., Ginanni Corradini S., Attili A. F., Capocaccia L. Biliary secretion of phosphatidylcholine and its molecular species in cholecystectomized T-tube patients: effects of bile acid hydrophilicity. Biochem Med Metab Biol. 1986 Oct;36(2):125–135. doi: 10.1016/0885-4505(86)90116-7. [DOI] [PubMed] [Google Scholar]
- Alvaro D., Angelico M., Cantafora A., Di Biase A., Gaeta G. B., Ginanni Corradini S., Tripodi M. F., Attili A. F., Utili R. Influence of tauroursodeoxycholic and taurodeoxycholic acids on hepatic metabolism and biliary secretion of phosphatidylcholine in the isolated rat liver. Biochim Biophys Acta. 1986 Sep 12;878(2):216–224. doi: 10.1016/0005-2760(86)90149-9. [DOI] [PubMed] [Google Scholar]
- Angelico M., Alvaro D., Masella R., Ginanni Corradini S., Cantafora A. Transport, utilization and biliary secretion of lysophosphatidylcholine in the rat liver. Biochim Biophys Acta. 1987 Nov 27;905(1):91–99. doi: 10.1016/0005-2736(87)90012-5. [DOI] [PubMed] [Google Scholar]
- BALINT J. A., KYRIAKIDES E. C., SPITZER H. L., MORRISON E. S. LECITHIN FATTY ACID COMPOSITION IN BILE AND PLASMA OF MAN, DOGS, RATS, AND OXEN. J Lipid Res. 1965 Jan;6:96–99. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Barnwell S. G., Tuchweber B., Yousef I. M. Biliary lipid secretion in the rat during infusion of increasing doses of unconjugated bile acids. Biochim Biophys Acta. 1987 Nov 21;922(2):221–233. doi: 10.1016/0005-2760(87)90158-5. [DOI] [PubMed] [Google Scholar]
- Borgström B., Krabisch L., Lindström M., Lillienau J. Deconjugation of bile salts: does it occur outside the contents of the intestinal tract in the rat? Scand J Clin Lab Invest. 1987 Oct;47(6):543–549. doi: 10.1080/00365518709168466. [DOI] [PubMed] [Google Scholar]
- Coleman R. Biochemistry of bile secretion. Biochem J. 1987 Jun 1;244(2):249–261. doi: 10.1042/bj2440249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardison W. G., Apter J. T. Micellar theory of biliary cholesterol excretion. Am J Physiol. 1972 Jan;222(1):61–67. doi: 10.1152/ajplegacy.1972.222.1.61. [DOI] [PubMed] [Google Scholar]
- Hardison W. G., Evans W. H. Postobstructive subcellular organelle and biliary lipid composition in the rat. A selective increase in biliary lecithin output is not reflected by changes in organelle composition. J Hepatol. 1986;3(3):318–327. doi: 10.1016/s0168-8278(86)80484-6. [DOI] [PubMed] [Google Scholar]
- Hostetler K. Y., Reasor M., Yazaki P. J. Chloroquine-induced phospholipid fatty liver. Measurement of drug and lipid concentrations in rat liver lysosomes. J Biol Chem. 1985 Jan 10;260(1):215–219. [PubMed] [Google Scholar]
- Kawamoto T., Okano G., Akino T. Biosynthesis and turnover of individual molecular species of phosphatidylcholine in liver and bile. Biochim Biophys Acta. 1980 Jul 14;619(1):20–34. [PubMed] [Google Scholar]
- Kempen H. J., Kuipers F., van Berkel T. J., Vonk R. J. Effect of infusion of "tris-galactosyl-cholesterol" on plasma cholesterol, clearance of lipoprotein cholesteryl esters, and biliary secretion in the rat. J Lipid Res. 1987 Jun;28(6):659–666. [PubMed] [Google Scholar]
- Kuipers F., Havinga R., Bosschieter H., Toorop G. P., Hindriks F. R., Vonk R. J. Enterohepatic circulation in the rat. Gastroenterology. 1985 Feb;88(2):403–411. doi: 10.1016/0016-5085(85)90499-8. [DOI] [PubMed] [Google Scholar]
- Leyton J., Drury P. J., Crawford M. A. In vivo incorporation of labeled fatty acids in rat liver lipids after oral administration. Lipids. 1987 Aug;22(8):553–558. doi: 10.1007/BF02537280. [DOI] [PubMed] [Google Scholar]
- Robins S. J., Brunengraber H. Origin of biliary cholesterol and lecithin in the rat: contribution of new synthesis and preformed hepatic stores. J Lipid Res. 1982 May;23(4):604–608. [PubMed] [Google Scholar]
- Roerdink F., Regts J., Van Leeuwen B., Scherphof G. Intrahepatic uptake and processing of intravenously injected small unilamellar phospholipid vesicles in rats. Biochim Biophys Acta. 1984 Mar 14;770(2):195–202. doi: 10.1016/0005-2736(84)90130-5. [DOI] [PubMed] [Google Scholar]
- Setchell K. D., Worthington J. A rapid method for the quantitative extraction of bile acids and their conjugates from serum using commercially available reverse-phase octadecylsilane bonded silica cartridges. Clin Chim Acta. 1982 Oct 27;125(2):135–144. doi: 10.1016/0009-8981(82)90190-5. [DOI] [PubMed] [Google Scholar]
- Spanjer H. H., van Galen M., Roerdink F. H., Regts J., Scherphof G. L. Intrahepatic distribution of small unilamellar liposomes as a function of liposomal lipid composition. Biochim Biophys Acta. 1986 Dec 16;863(2):224–230. doi: 10.1016/0005-2736(86)90262-2. [DOI] [PubMed] [Google Scholar]
- Stoffel W. Chemical synthesis of choline-labeled lecithins and sphingomyelins. Methods Enzymol. 1975;35:533–541. doi: 10.1016/0076-6879(75)35181-1. [DOI] [PubMed] [Google Scholar]


