Abstract
Introduction:
Fungal infections are caused by a broad range of pathogenic fungi that are found worldwide with different geographic distributions, incidences and mortality rates. Considering that there are relatively few approved medications available for combating fungal diseases and no vaccine formulation commercially available, multiple groups are searching for new antifungal drugs, examining drugs for repurposing and developing antifungal vaccines, in order to control deaths, sequels and the spread of these complex infections.
Areas covered:
This review provides a summary of advances in fungal vaccine studies and the different approaches under development, such as subunit vaccines, whole organism vaccines, and DNA vaccines, as well as studies that optimize the use of adjuvants. We conducted a literature search of the PubMed with terms: fungal vaccines and genus of fungal pathogens (Cryptococcus spp., Candida spp., Coccidioides spp., Aspergillus spp., Sporothrix spp., Histoplasma spp., Paracoccidioides spp., Pneumocystis spp. and the Mucorales order), a total of 177 articles were collected from database.
Expert opinion:
Problems regarding the immune response development in an immunocompromised organism, the similarity between fungal and mammalian cells and the lack of attention by health organizations to fungal infections are closely related to the fact that, at present, there are no fungal vaccines available for clinical use.
Keywords: Fungal vaccine, Prophylactic vaccine, Subunit, Therapeutic vaccine
Graphical Abstract
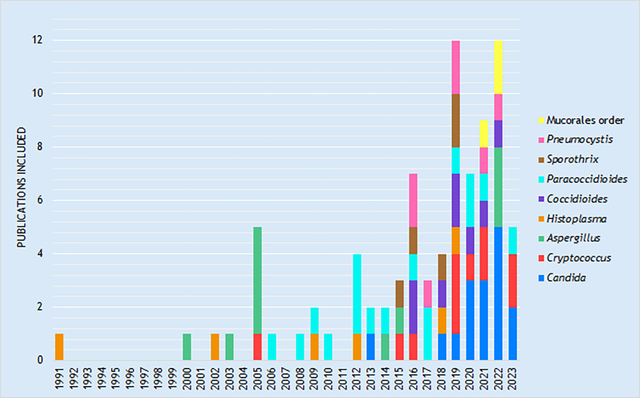
Graphic 1. Distribution of selected articles by fungus and year of publication included in the compilation.
1. Introduction
Fungal infections occur worldwide, and their distribution and incidence vary according to characteristics of the regions, such as geography, weather and the level of social development. In their first ever Fungal Priority Pathogens List, the World Health Organization (WHO) ranked several fungal pathogens according to their epidemiology, such as annual incidence, lethality, effectiveness of treatment and global distribution, defining Cryptococcus neoformans, Candida auris, Aspergillus fumigatus and Candida albicans as the critical group. Other Candida species, such as C. parapsilosis, C. glabrata (now Nakaseomyces glabrata), and C. tropicalis were recognized in the high priority group, together with Histoplasma spp., Fusarium spp., Murorales, and Eumycetoma causative agents. Certain other fungi associated with pulmonary infections were placed in the medium group, such as Pneumocystis jirovecii and Paracoccidioides spp. [1]. Notably, other major fungi, such as Sporothrix brasiliensis that is causing an ongoing epidemic in Brazil, were not recognized, underscoring that there are additional fungi of great import that are also worthy of recognition that were omitted from the FPPL.
In 2019, it was estimated that the direct medical costs in the United States (US) were due to fungal infections was $ 7.5 billion. In 2021, ~7,199 deaths in the US were associated with fungal diseases [2] and this increased during the COVID-19 pandemic to ~13,121 [3]. Globally, there are over 1.7 million deaths estimated as being due to fungal diseases [4]. These data reinforce the profound impact of fungal infections worldwide and, consequently, the current need to develop new approaches in order to control these threats. This is especially significant as there are increasing reports of drug resistance in species including C. neoformans [5], C. albicans [6], A. fumigatus [7], H. capsulatum [8] and Sporothrix brasiliensis [9] and there is innate antifungal resistance to current drugs in other fungi, such as Candida auris [10] and fungi from Mucorales order [11].
There are numerous efforts underway to bypass treatment challenges, such as the development of new antifungal drugs [12], drug repurposing [13] and antifungal vaccine discovery. Vaccines are the most successful biomedical advance in preventing disease, avoiding between 2 and 3 million deaths annually [14]. Vaccine approaches, including therapeutic and prophylactic protocols, are a valuable way to control infectious diseases since vaccines have diverse health impacts, reducing deaths and the development of disabling disease forms, and they may reduce disease transmission. Vaccines also have the capacity to eradicate diseases. They also have an important economic impact, including cost savings [15]. Table 1 and Figure 1 present an overview of many efforts related to the development and advancement of fungal vaccines.
Table 1.
Advances in fungal vaccines
| FUNGI (REFERENCE) | VACCINE TYPE | ADJUVANTS | MECHANISM OF IMMUNE STIMULATION |
|---|---|---|---|
| Candida spp. [23, 25, 27 – 31, 34 – 41] | NDV-3A and NDV-3/Subunit vaccine | Aluminum hydroxide in phosphate-buffered saline | Opsonophagocytosis killing |
| Sap2 recombinant proteins | Alum | Induce IgG and IgM antibodies and Th1/Th2/Th17 cytokine | |
| C. albicans gpi7/avirulent vaccine | - | Induce B220+CD44+CD138+MHCII-LLPCs and IL-18 | |
| Extracellular vesicles (EVs) | Freund’s adjuvant | Increase IL-6, IgM, IL-12p70, TNFα and IFNγ | |
| PCA2/low-virulence vacinne | - | Mobilized HSPCs and increased TNF-α and IL-6 | |
| Avirulent Candida strains | - | Mediated by Ly6G1 Gr-11 putative granulocytic MDSCs | |
| NDV-3A/Subunid vaccine | Freund’s adjuvant | Opsonophagocytosis killing/Anti-Als3p antibodies and CD4+ T helper cells activating tissue macrophages | |
| Hyr1 (rHyr1p-N)/Subunit vaccine | Freund’s adjuvant (CFA)/incomplete Freund’s adjuvant (IFA) | Induce neutrophil killing activity | |
| MHC class-II epitopes | - | Induce IgM | |
| Polybacterial MV140 + C. albicans V132 | - | Induce human Dendritic Cells and promotes a potent proinflammatory cytokine production | |
| Nanoparticles (NPs) + Hsp 90-CTD | Polyethyleneimine (PEI) | Inducted differentiation of B cells | |
| Secreted aspartyl proteinases (Saps)/multi-epitope vaccine (MEV) | RS09 (APPHALS) and the N and C terminals of Salmonella dublin flagellin protein | Shows stably connect with MHC-I and Toll-like receptor molecules | |
| IVIG immunoglobulin | - | Shows higher titers of Candida-specific IgGs | |
| Cryptococcus spp. [45, 47, 49, 51, 54 – 56, 58, 60 – 63] | GXM / Subunit vaccine | tetanus toxoid | developed protective antibodies against GXM and reduced lung fungal burden |
| Live and HK Cn strain overexpressing ZNF2 | - | Protective Th1 and Th17 immune response | |
| Cn expressing IFN-γ | - | Trained immunity of DCs | |
| HK Cg mutant Δcap60 | DC-pulsed | Protection and induction of memory-resident Th17 cells in the lungs | |
| Live or HK Cn mutant Δcda1/2/3 | - | Protective Th1-type adaptive immune response | |
| HK Cn fbp1Δ mutant | - | Cross-protection against Multiple Invasive Mycoses | |
| GP-Cda1 and GP-Cda2 | GP | Th1 and Th17 responses | |
| Cda2 antigen | TEOS and GP | Th1 and Th17 responses | |
| Aspergillus spp. [68 – 73, 76, 77, 80, 81] | Crude culture filtrate or Asp f2/Crude or subunit vaccine | - | Protective Th1 memory responses |
| RNA or live fungi/Crude or subunit vaccine | human and murine DC-pulsed (RNA was complexed with DOTAP*) | DCs shows increase of MHC-II, costimulatory receptors and Th1/Th2 response | |
| Glycoconjugate of β-1,3-D-glucan/Subunit-panfungal vaccine | Diphtheria toxoid | Antibodies opsonize cells and complement system | |
| Live or HK conidia/Crude vaccine | - | Live conidia shows Th1 response and specific antibodies. Heat-killed conidia shows no response | |
| A9 mAb (IgG1 of cell wall antigen of A. fumigatus)/mAb therapy | - | Opsonophagocytic killing | |
| HK fungi/Crude vaccine | human DC-pulsed | In vivo protective Th1 response (high IFN-g/low IL-10) with specific T cells | |
| HK yeast of S. cerevisiae/Crude vaccine | - | Specific Th1 response | |
| Culture filtrate, Asp f5 or Asp f13/Crude or subunit vaccine | - | Increase of airway hyper-responsiveness, IgE and Th2 allergic response | |
| Asp f3 and Asp f9/Subunit vaccine | Lipated Tucaresol | IgG1 antibodies and increase of IL-4 | |
| A. fumigatus ΔsglA mutant live or HK conidia/Crude vaccine | - | Suggesting of proinflammatory immune response. Live or HK increased animal survival | |
| NXT-2/Subunid-panfungi vaccine | - | Opsonophagocytic killing | |
| Histoplasma spp. [85, 87 – 89, 91, 92] | Hc protein extract | - | Protective immune response |
| mAb Hsp60 | - | Protective against lethal infection | |
| mAbs M antigen | - | Reduced lung fungal burden | |
| Hc alkaline extract | GPs | Reduced lung fungal burden and increased Th1 and Th17 responses | |
| rHsp60 glycoprotein | - | Induced specific reactive CD4+ or CD8+ T cells | |
| Coccidioides spp. [106, 108 – 111] | Δcts2/ard1/Δcts3/Live attenuated vaccine | - | Increased Th1 and Th17 responses and innate immune cells recruitment |
| Δcps1/Live attenuated vaccine | - | Reduced lung fungal burden | |
| rCpa1/Subunit vaccine | GCP | Increased Th17 response | |
| Ag2/Subunit vaccine | GCP | Increased Th17 response | |
| Ag2/Subunit vaccine | DC-pulsed | Enhanced IgG levels | |
| Ag2/Subunit vaccine | GCP | Enhanced IgG levels and reduced lung fungal burden | |
| Δcps1/Live attenuated vaccine | - | Reduced lung fungal burden in dogs prime- and boost-vaccinated | |
| Δcps1/Live attenuated vaccine | - | Reduced lung fungal burden | |
| Paracoccidioides spp. [118, 120, 122 – 125, 127, 130 – 134, 137, 138, 143 – 145] | P10 + Antifungals | Freund’s adjuvant | Th1 and Th2 response |
| gp43 or P10 | FliC flagelin | gp43 + FliC increased fungal burden. P10 + FliC promoted Th1 response | |
| P10 + Antifungals | PLGA | Increased of Th1 response with decreased of IL-10 | |
| P10 | Allum, FliC flagelin, cationic lipids, Freund’s adjuvants | All adjuvants increased levels of IFN-g but a few ones decreased IL-10 | |
| pcDNA3-pP10 | pIL-12 | Increased specific antibodies and memory CD4+ T cells | |
| MAb 3E | - | Reduced fungal burden and decreased lung inflammation | |
| Hsp60 and 7B6 mAbs | - | Opsonophagocytic killing | |
| GSL pAbs | - | Activation of macrophages by IFN-g and increase of phagocytic activity | |
| P10 | VLP from HepB | specific CD4+ T cells with less IL-10 levels | |
| P10 | DC-pulsed | Th1/Th17 response pattern | |
| P10 + Antifungals | cationic lipids DDA/TDB* | Th1 /Th17 response pattern | |
| F1.4 mAbs + antifungals | - | Opsonophagocytic killing | |
| P10 | Chitosan nanoparticules | Th1/Th17 response pattern | |
| Sporothrix spp. [150, 158, 159, 162, 163] | P6E7/mAb therapy | - | Reduced fungal burden in earlier infection |
| ssCWP (CWP100 and CWP10)/subunit vaccine | AH | Increased Th1, Th2 and Th17 responses ex vivo and stimulated passive protection in non-immunized mice | |
| ZR8, ZR4 and ZR3/subunit vaccine | - | Increased Th1 and Th17 responses and B cells (especially ZR8) | |
| CWP100/subunit vaccine | AH and PGA | Association with PGA demonstrated greater IgG levels and Th1-major response | |
| rSsEno | PGA | Greater IgG levels and increased Th1 and Th2 responses | |
| Pneumocystis spp. [165 – 169, 171, 172] | p55-TAG DNA vaccine | - | Induce IFN-γ and IL-17 response |
| Pneumocystis Live vaccine | - | Induce the CD4+ T cells, CD8+ T cells, CD19+ B cells, and CD11b+ macrophages | |
| Pca1/recombinant protein | TiterMax Gold | Induce antigen-specific antibody | |
| GSC-1 ectodomain/Subunit vaccine | Imject alum | Antibody response | |
| A121–85/recombinant antigen | PBS | Increased the IgG, promoted the secretion of T lymphocytes, increased the expression of inflammatory factors | |
| KEX1 protein | Aluminum hydroxide | Induce humoral responses and B cell memory | |
| Mucorales Order (in silico and in vitro) [176, 177] | Multitope vaccine | beta-defensin | Stable TLR-2 binding |
| FTR1 protein based vaccine | RS09 and beta-defensin-3 | Increased of active B and T cells along with elevated levels of different immunoglobulins | |
| Multitope vaccine | L7/L12 50S ribosomal protein | Interactions with MHC-I, TLR2, and GRP78 with a innate and adaptive cells responses |
Figure 1.
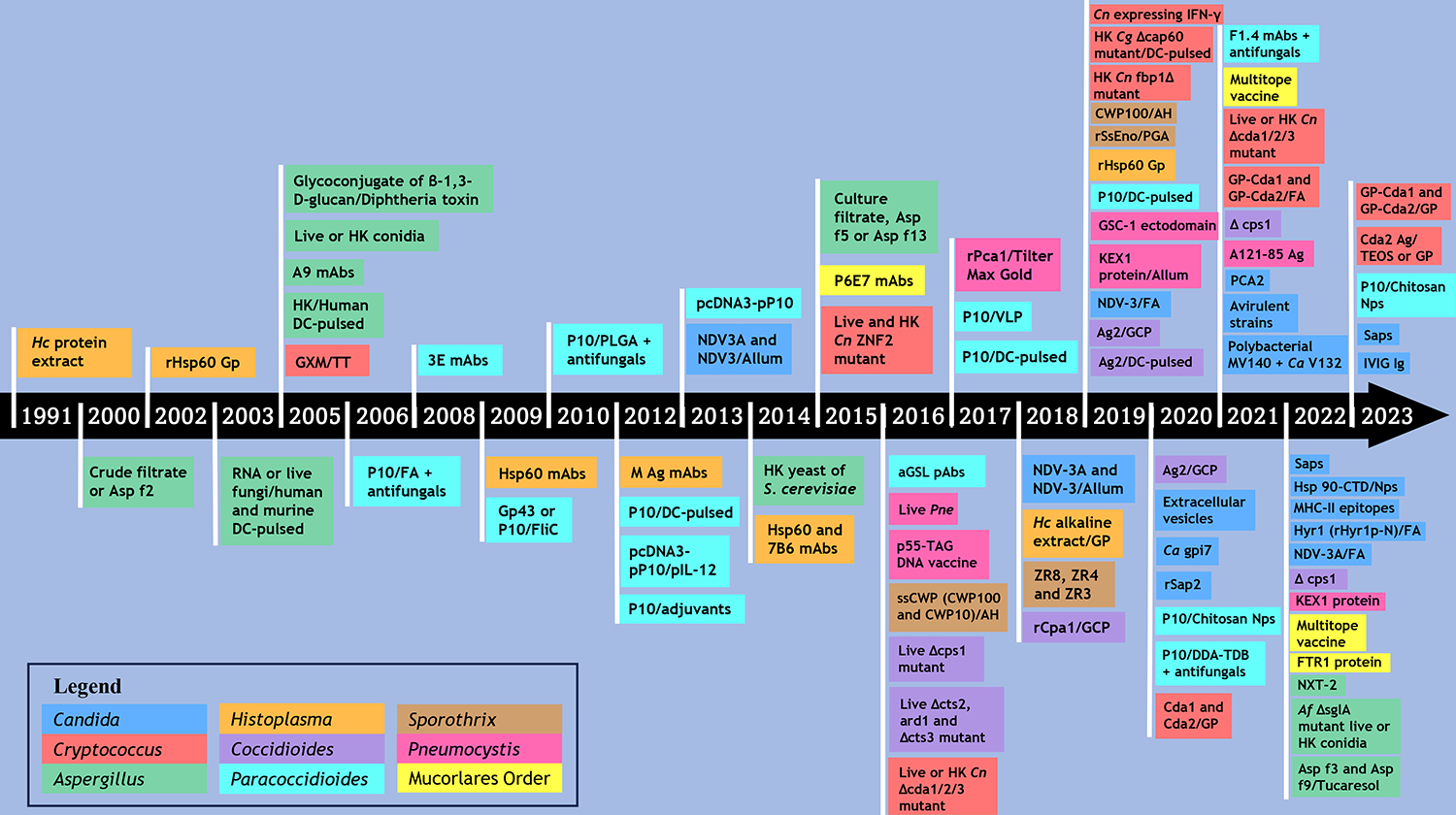
Timeline of Fungal Vaccine Approaches. The timeline is based on the compilation: (aGSL: acidic glycosphingolipids; AH: aluminum hydroxide; Ca: C. albicans; Cg: C. gattii; Cn: C. neoformans; CWP: cell wall proteins; DC: dendritic cell; DDA-TDB: dioctadecyldimethylammonium with a trehalose dibehenate-cationic adjuvant formulation; DOTAP: cationic lipid N-[1-(2,3-dioleoyloxypropyl]-N, N, N,-trimethylammonium methylsulfate; FA: Freund’s adjuvant; GCP: glucan-chitin particles; gp: glycoprotein; GLP: glucan particles; GSC-1: Pneumocystis catalytic subunit of β-1,3-glucan synthase; GXM: glucuronoxylomannann; Hc: H. capsulatum; HK: Heat-killed; IVIG: Intravenous immunoglobulin; KEX1: Kexin 1 protein; mAbs: monoclonal antibodies; MHC-II: Major Histocompatibility Complex Class II; NDV-3: Als3 fused to a 6-His tag and linker sequences; NDV-3A: Als3 with no extraneous sequences; Nps: nanoparticles; NXT-2: a panfungal peptide based on homologous sequences from Pneumocystis, Aspergillus, Candida, and Cryptococcus; PGA: Montanide™ Pet Gel A; PLGA: Poly (lactic acid-glycolic acid); Pm: P. murina; Saps: secreted aspartyl proteinases; ssCWP: S. schenckii yeast cell wall proteins; TEOS: tetraethylorthosilicate; TT: Tetanus toxoid; VLP: virus-like particles.)
The similarity between fungal and mammalian cells, inadequate immune response in immunocompromised hosts, fungal neglect, and lack of attention from health agencies are some of the factors that present disadvantages to the development of vaccines. Our review highlights the important contributions and efforts achieved in recent years in the study of therapeutic and/or prophylactic vaccines against fungal diseases. These data provide an overview of research in the area and discuss the difficulties, opportunities and future directions related to these studies. We discuss recent studies on the search for vaccine candidates focusing on Candida spp, Coccidioides spp, C. neoformans, Aspergillus spp, Sporothrix spp, Histoplasma spp, Paracoccidioides spp, Pneumocystis jirovecii, and the order Mucorales.
2. Vaccine and Pathogenic Fungi
Studies for the development of fungal vaccines have continued to advance over the years with promising candidates and adjuvants. Below we present the results of investigations into the genera of fungi that are highlighted in the topic of vaccines.
2.1. Candida spp.
About 15.5 million cases of oral candidiasis and 700,000 cases of invasive candidiasis occur annually [16]. Almost 95% of Candida spp. infections are attributed to C. albicans, C. tropicalis, C. parapsilosis, C. glabrata, C. krusei and C. auris [16–18]. Invasive candidiasis has been associated with reduction in the susceptibility of the polyenes, azoles and echinocandins, which supports new approaches to control these fungi. Synthetic vaccines have shown promise against Candida infections [19].
Subtractive proteomic approaches have been used for selection of antigens for designing Candida vaccines. In Candida spp. immunodominant cell wall proteins: agglutinin like sequence 3 (Als3), secreted aspartyl proteinases (Saps), and hyphally regulated gene 1 (Hyr1) have been investigated as vaccine candidates, and the results have been promising, showing protection against infections [20–22].
The recombinant Als3 antigen, NDV-3A (Als3 with no extraneous sequences) and NDV-3 (Als3 fused to a 6-His tag and linker sequences) have similar safety and elicited robust immunologic responses against C. albicans [23,24]. In addition, NDV-3 and NDV-3A protect mice from vulvovaginal candidiasis (VVC) due Anti-Als3p-N acting as opsonin to increase IFN-γ primed neutrophil function in killing C. albicans [23,25].
Edwards and co-workers [23], conducted the first clinical trial phase 1b/2a of a vaccine against recurrent vulvovaginal candidiasis (RVVC). Overall, in 178 women with RVVC, one intramuscular dose of NDV-3A provide robust B- and T-cell immune responses. This therapeutic vaccine reduced the symptomatic episode and increases median time to recurrence under women aged <40 years at 12 months post-vaccination. Alqarihi et al [24] highlight that NDV-3A vaccination significantly reduces C. albicans virulence both in vitro and in vivo. The vaccine with NDV-3A elicits antibody responses that attenuate C. albicans virulence as demonstrated by decreased C. albicans adherence, biofilm formation, and invasion of mucocutaneous barriers [26]. The NDV-3A vaccine also may be a strategy to prevent Candida infections on indwelling medical devices, such as central venous catheters. Thus, NDV-3A vaccination not only mitigates diseases, but can also prevent the source of dissemination [24].
The Als3 adhesin protein is also a target for vaccine development against C. auris [27]. The NDV-3A vaccine enhances opsonophagocytic killing of C. auris by macrophages as well as augmenting CD4+T cell immune responses. Moreover, NDV-3A vaccination improves the activity of micafungin and protects mice from lethal C. auris infections [28]. The Als3 (rAls3p-N) and Hyr1 (rHyr1p-N) from C. albicans administered with Freund’s adjuvant (FA)/incomplete Freund’s adjuvant effectively decreases fugal burdens in the kidneys of mice pups [20]. A monoclonal antibody (mAb) to Hyr1p has also been shown to protect against systemic C. auris disease in mice [29].
Eight epitopes from C. dubliniensis Saps with antigenic, non-toxic and non-allergenic properties have immunogenic potential against C. dubliniensis infections as their injection in mice increased the total numbers of plasma B-lymphocyte, B-cell and Th-cell as well as total antibody levels, after the second and third doses [22]. For C. tropicalis, vaccination with Sap2 enhances cell-mediated and humoral immune responses through engagements with MHC-I and Toll-like receptors [21]. Vaccination with Sap2p from C. parapsilosis increases IgG and IgM antibodies and Th1/Th2/Th17 cytokines, and is protective against C. tropicalis systemic candidiasis [30].
Gupta and co-workers [31] selected two MHC class-II epitopes (QTTCFQTEYYDPYIS and FVDPKKCCCDPKMIK) from C. auris, with antigenicity scores higher than 0.5 [31]. Vaccination with these epitopes increased IgM levels after the second injection, followed by long-term immune response up to 6 months, suggesting that such vaccines have the potential to treat C. auris infections.
A secretome analysis of C. glabrata revealed 33 candidate secretory proteins with immunogenic properties, and immunizations with these antigens resulted in reductions in the fungal burden on the liver, spleen, kidney, and lungs of mice infected with the fungus. This work highlights that C. glabrata secretory proteins are potential agents for antifungal vaccines development [32]. C. auris protein and peptide targets also showed cell-mediated and humoral immune responses, and can be used in the design of multi-epitope vaccine candidates (MEVCs) [33].
Another vaccination strategy uses the C. albicans PCA2 (a low-virulence, non-germinative C. albicans), and administration of this strain resulted in the mobilization of the spleen to form myeloid cells trained hematopoietic stem progenitor cells (HSPCs), which promoted high pro-inflammatory cytokine responses and increased TNF-α and IL-6. Vaccination with PCA2 protected mice against a lethal secondary infection, with lower fungal burdens in the kidneys and the spleen [34]. Vaccination with low-virulent or avirulent Candida strains can also modify innate immunity. Vaccination with the avirulent C. albicans glycosylphosphatidylinositol (gpi)7 mutant mediated innate immune responses and adaptive humoral immunity against infections caused by C. albicans and non-albicans Candida spp. Vaccination with the C. albicans gpi7 mutant increased the number of B220+CD44+CD138+MHCII-LLPCs in spleens and the bone marrow. The β-(1,3)-glucan from the C. albicans gpi7 mutant vaccine triggers the Dectin-1 dependent activation of non-canonical NF-kB subunit RelB resulting in higher IL-18 expression [35]. Furthermore, intraperitoneal vaccination by a low-virulent C. dubliniensis confers protection to lethal challenge by C. albicans and Staphylococcus aureus, which is mediated by Ly6G1 Gr-11 putative granulocytic myeloid-derived suppressor cells (G-MDSCs) [36].
The combination of polybacterial MV140 (25% Escherichia coli, 25% Proteus vulgaris, 25% Klebsiella pneumoniae and 25% Enterococcus faecalis) and C. albicans V132 represents a vaccine formulation with promising activity for controlling recurrent urinary tract infections (RUTIs) and RVVCs. The MV140/V132 vaccine increases dendritic cells (DCs) maturation, CD80 proinflammatory cytokines and IL-10. These DCs activate an increased production of mitogen-activated protein kinases (MAPK), nuclear factor-kB (NF-kB) and the mammalian target of rapamycin (mTOR)-mediated signaling pathways [37]. The C. albicans V132 sublingual vaccine improves glycolysis and oxidative phosphorylation and epigenetic reprogramming transcriptional pro-inflammatory genes IL6 and TNF-α, and also mediates IL-17 responses after in vitro stimulation with MV140 [38].
Nanoparticles are a promising strategy for the development of Candida vaccines. These nanostructures constituted by heat shock protein (Hsp) 90-CTD combined with polyethyleneimine (PEI), which forms a stable and efficient antifungal nanovaccine. A PEI nanovaccine induced faster antibody production by antigen presentation to mature B cells and inducted the differentiation of B cells into long-lived plasma cells (LLPCs) in the bone marrow, producing long-lasting immunological protection against C. albicans infections [39].
Extracellular vesicles (EVs) are lipid bilayer compartments formed by diverse species, including fungi such as Candida, are also recognized as a vaccine platform. Immunization with EVs from C. albicans decreased the fungal burden of lethally challenged mice in their spleens and livers, while increasing the IL-6 and IgM. The mice vaccinated with EVs and FA induced higher levels of IL-12p70, TNF-α and IFN-γ. Another noteworthy observation is that the pretreatment with C. albicans EVs prolonged the survival of Galleria mellonella subsequently infected with C. albicans [40].
Intravenous immunoglobulin (IVIG) has been a supplementary strategy for sepsis and septic shock treatment. A new IVIG is being studied for treatment and prevention of disseminated candidiasis by C. auris or C. albicans. IVIG with high-titer IgGs specific for Candida cell epitopes was more efficient in reducing the fungal burden and prolonged survival in mice infected with either of these yeast species. IVIG batch 1741 combined with amphotericin B (AmpB) enhanced the therapeutic efficacy of the polyclonal antibody (pAb) mixture against C. auris and C. albicans invasive infections, and was also effective at a decreased AmpB dosage [41].
2.2. Cryptococcus spp.
In 2020, ~152,000 cases of HIV-associated cryptococcal meningitis were estimated worldwide, resulting in ~112,000 deaths [42]. As the global burden of cryptococcosis remains most prevalent in immunocompromised individuals, it is essential that studies of new therapeutic options, such as the development of vaccines, be performed using models that mimic immunodeficiency, as it is necessary to know the cell subtypes that may be associated with protection and containment of infection in these conditions.
The capsule of Cryptococcus spp. is the main virulence factor of the species, and, among the fungi of medical importance, it is the only encapsulated one. In terms of vaccine development, the polysaccharide capsule presents challenges and advantages. The first experimental vaccine against C. neoformans was composed of the major polysaccharide antigen from the pathogen’s capsule, known as glucuronoxylomannann (GXM) [43]. GXM does not require T cell help for the generation of an antibody response, but GXM conjugated to tetanus toxoid was developed to improve the antigenicity of the vaccine. Vaccinated mice developed antibodies against GXM after a challenge with C. neoformans, and some of these antibodies were partially protective [44,45]. Similarly, protective antibodies were induced in a study performed with a GXM-conjugated mimetic peptide [46].
Several techniques for vaccine discovery have focused on protein antigens that stimulate the response of T cells. Cryptococcal vaccine development efforts have integrated whole-organism approaches that are attenuated by the deletion of important virulence factors such as cell wall chitosan, the capsular polysaccharide GXM, F-box protein, and sterylglycosidase [47–52]. In recent work, the search is more directed on individual and specific antigens in the protection of mouse models with cryptococcosis [53–56].
Two whole yeast Cryptococcus vaccine approaches are worth specifically highlighting. In the first approach, a C. neoformans strain genetically engineered to express murine IFN-γ was used to vaccinate mice, and the mice vaccinated with the genetically engineered strain were fully protected against a wild-type strain. The protection was achieved by induction of trained immunity of DCs and the mechanism was dependent on STAT1 signaling [57,58]. Another approach used heat-killed acapsular C. gattii cells to pulse bone marrow-derived DCs that were then injected intravenously into mice. The vaccine resulted in protection and induction of memory-resident Th17 cells in the lungs of recipient mice infected with a virulent strain of C. gattii [59].
Additional vaccine candidates based on protection data with heat-killed cryptococcal and live-attenuated mutant strains have been evaluated. One strain constructed by deletion of three chitin deacetylase genes, an avirulent chitosan-deficient strain, elicited a protective Th1-type adaptive immune response that conferred full protection against lethal infection by C. neoformans [47]. Vaccinations with other mutant C. neoformans strains, such as a F-box protein Fbp1 deletion mutant [49] or a strain overexpressing the transcription factor Znf2 [60], also protected mice against lethal challenges [52].
Whole-organism vaccines generally elicit strong immune responses and are relatively easy to develop. However, there are important disadvantages, which include autoimmunity and potential for reactogenicity, and, for live vaccines, infection. Studies focused on the identification of C. neoformans protein antigens have been advancing for the generation of subunit vaccines. Chitosan, a form of chitin deacetylated (Cda), is a necessary factor for virulence in C. neoformans. Two members of the Cda family, Cda1 and Cda2, are promising vaccine antigens. In addition to their role in catalyzing chitin deacetylation, these antigens have strong immunogenicity and do not show significant homology with human proteins [61]. Positive results have been obtained with glucan particle (GP)-based subunit vaccines, including GP-Cda1 and GP-Cda2, alone and in combination, with both demonstrating enhanced mouse survival after challenge with the virulent C. neoformans KN99 strain [54,62]. A more recent study evaluated the protection mediated by the GP vaccine, GP-Cda1 and GP-Cda2, in mice with specific deficiencies in immune function, including congenital and acquired deficiencies. Robust and long-lasting Th1 and Th17 immune responses detected in the lungs were observed in vaccinated and infected mice [55].
New formulations to encapsulate proteins and/or peptides in order to keep these molecules stable are being investigated for vaccine development. Soto and co-authors presented results of an encapsulation approach of the Cda2 antigen using tetraethylorthosilicate (TEOS), which confers thermal stability to the protein inside the hollow cavity of GPs (Glucan particles) [56]. The GP-Cda2 ensilicated vaccine protected vaccinated mice from a lethal pulmonary infection of C. neoformans [56,63].
The species of C. neoformans and C. gattii induce different immune responses and distinct clinical manifestations, and they also differ in the expression of proteome in infections. Candidate vaccines capable of protecting against both species would be ideal for advancing to human trials.
2.3. Aspergillus spp.
Aspergillus are ubiquitous saprophytic fungi in the environment that cause diverse health problems ranging from allergic responses to life-threatening infections. There are about 200 cataloged species of the genus, and less than 20 are pathogenic [64,65]. A review by Levitz [66] discusses that experimental aspergillosis vaccines can be divided into four categories: pan-fungal, crude, subunit, and therapeutic [66].
Pan-fungal vaccines target shared antigens among medically important fungal species with the concept of inducing protection against many different mycoses. One example is a conjugate glycoprotein of β-1,3-D-glucan with diphtheria toxoid, which has demonstrated efficacy in safeguarding mice from infections caused by both C. albicans and A. fumigatus [67]. Another recent study published a pan-fungal peptide discovered based on homologous sequences of Pneumocystis, Aspergillus, Candida and Cryptococcus, called NXT-2, which reduces morbidity and mortality in animal models of invasive fungal infections by these species. This vaccine is highly immunogenic and appears to function by binding to the surface of conidia and hyphae, promoting opsonophagocytic killing. Hence, NXT-2 is a promising candidate for further exploration [68].
On the other hand, crude vaccines consist of whole or fractionated Aspergillus, but concerns about autoimmune responses and reactogenicity may limit future human testing. Nevertheless, vaccination with live A. fumigatus or crude culture filtrates can significantly protect against intranasal or intravenous challenge with A. fumigatus conidia [69,70], although this approach can also trigger a Th2 response. There have been variable responses when using the whole fungus, heat-killed solutions, protein filtrates, and even live fungus in murine experiments. In certain studies, mice immunized with heat-killed Aspergillus generated a non-protective response, while mice immunized with live conidia demonstrated high titers of protective antibodies that correlated with a protective response against lethal challenge [71,72]. However, another study using a different heat-killed protocol demonstrated that the vaccinated mice survived to the experimental endpoint [73]. The mechanism of this protection is not yet fully elucidated, but it is suspected that live conidia end up growing and forming filamentous forms in the tissue, which, upon contact with the innate immune system, end up being more reactive and generating more efficient responses [71]. Moreover, the defense against aspergillosis does not solely rely on antibodies, and it has been suggested that the mouse strain used in the study can make up for the lack of certain immunoglobulin classes. More studies are needed in different animal models to further conclusions [68,72]. Interestingly, live mutant conidia (A. fumigatus with the steryl glucosides-encoding gene deleted) that accumulate glycolipids are not capable of causing disease when injected into murine lungs. Moreover, animals previously challenged with live mutant conidia are protected against subsequent infection with live wild-type conidia, even in sublethal doses. Furthermore, the protection from a secondary challenge also occurs following vaccination with heat-inactivated mutant conidia [73].
Subunit vaccines, which contain purified components of Aspergillus, have also been tested in preclinical models. Vaccination with individual recombinant proteins from Aspergillus, including peroxiredoxin (Asp f3), 1,3-beta-glucanosyltransferase (Gel1), extracellular cell wall glucanase Crf1 (Asp f9) and aspartic protease (Pep1), induce protection against aspergillosis, and purified cell wall glycans and cell wall mannans have also been used as immunogens [68,74,75].
Vaccination with A. fumigatus surface antigens, Asp f3 and Asp f9 (VesiVax Af3/9R) in association with lipidated Tucaresol protects mice with neutropenia or steroid-induced immune suppression against infections caused by different strains of A. fumigatus, including azole resistant strains. The vaccine-induced protection is linked to elevated levels of IL-4 secretion by splenocytes and increased concentrations of IgG1 antibodies against Aspergillus antigens, which are detectable three days following the last vaccine boost [76]. Along this line, passive administration of mAbs targeting A. fumigatus cell surface antigens can prolong the survival of mice challenged with A. fumigatus, demonstrating that vaccine-generated antibodies can confer protection [77,78]. Similarly, mAbs to A. flavus cell wall antigens have been generated and may be useful in diagnostics and/or therapeutics [79].
Dendritic cells (DCs) pulsed with Aspergillus conidia and conidial RNA can protect in murine infection models of hematopoietic transplantation, but depending on the antigen used, it is speculated that the adaptive response generated would not be specific [80–83]. However, there are many obstacles to implementing DC-based therapies, including the high cost involved. Still, there has been a small clinical trial using DC-pulsed with antigens of A. fumigatus demonstrating protection against opportunist infections following haploidentical transplants in humans [81].
Although there are multiple types of vaccines for Aspergillus infections in development, numerous challenges remain. Vaccines for prophylaxis against infection with Aspergillus have been tested, but none have progressed to significant clinical trials [84]. Due to the constant exposure that the immune system has to fungal conidia present in the environment, approaches that prioritize antigens with greater specificity and immunogenicity are necessary to minimize antibody reactivity against non-specific proteins [66].
2.4. Histoplasma spp.
Histoplasma capsulatum is a dimorphic environmental fungus with worldwide prevalence. It is the causative agent of histoplasmosis, which is estimated to cause 250,000 to 500,000 new infections annually in the US, albeit clinical disease only occurs in a small fraction of these [85]. Although histoplasmosis is typically asymptomatic and self-limited in immunocompetent hosts, immunocompromised individuals (such as cancer and transplanted patients, HIV/AIDS patients, etc.) are at increased risk for severe and disseminated disease [86]. Because of its importance as a public health problem and the need for development of new methods of treatment that go beyond antifungal medication (e.g. amphotericin B and azoles), vaccination strategies are being developed.
In a pioneering study, vaccination of mice with the fungal cell wall and membrane extracts containing antigens that were recognized by T cells resulted in the triggering of protective immunity that was effective against a lethal challenge by H. capsulatum [87]. Subsequent studies focused on specific parts of the protective extract, which led to the creation of a rHsp60 glycoprotein vaccine [88]. Vaccination with rHsp60 induced specific reactive CD4+ or CD8+ T cells and the vaccine was effective in protecting mice against H. capsulatum [89]. However, the application of the rHsp60 vaccine was limited because of high homology with human Hsp60, which may lead to autoimmune reactions [90]. It is also notable that specific IgG1 isotype mAb to Hsp60 are protective against lethal infection [91]. Similarly, mAbs to the surface M antigen are protective [92]. In addition to this glycoprotein, vaccination with the H antigen, which is present on the surface of the fungus, also induced a robust immune response in murine models, but it did not protect mice against lethal challenge with H. capsulatum [93].
An alkaline extract from H. capsulatum was used as an immunogenic preparation for incorporation into glucan particles (GPs), and vaccination with these particles resulted in a protective response in mice. GPs effectively served as a system of adjuvant and antigen-presenting cell targeted delivery, resulting in the effective engagements of dectin-1 and complement receptor 3 (CD11b), and stimulating strong IL-17 and IFN-γ responses. The GPs reduce fungal burden by <80%, and improved survival rates in lethally infected mice [85]. The inclusion of additional compounds that modify immune responses onto the GPs could further enhance their efficacy. Subsequently, H. capsulatum peptide epitopes derived from Hsp60, enolase, and HSC82 proteins restricted to MHC-I and MHC–II were incorporated into GPs. The GPs with the peptides efficiently induced the proliferation of murine CD4+ and CD8+ T lymphocytes, and stimulated the production of IFN-γ and IL-17 [94]. As these highly promiscuous molecules are also expressed by several additional important fungal pathogens, this approach may be effective against fungal infections in addition to histoplasmosis.
Recently, using the whole genome sequencing of four H. capsulatum strains and reverse vaccinology, commonly shared proteins that were not homologous to the human host were identified by bioinformatic analysis. The method effectively identified essential H. capsulatum proteins, like β-1,3-glucanosyltransferase, that the study predicts as being promising candidates for a vaccine [95]. However, to date, the work has not been translated into an experimental vaccine.
Experimental vaccines against Histoplasma and reverse vaccinology technology present promising candidates to advance studies of immunizing agents for histoplasmosis. Targets such as the enzyme beta-1,3-glucanosyltransferase, involved in the elongation of beta-(1–3)-glucans, are found in several fungi and can be used in the development of cross-reactive vaccines with other fungal species [95].
2.5. Coccidioides spp.
Coccidioidomycosis, also known as San Joaquin Valley fever or Valley fever, is a fungal infection caused by Coccidioides posadasii and Coccidioides immitis. Driven by climate change, coccidioidomycosis has re-emerged as a major threat to human health such that in 2019 ~ 20,003 cases were reported to the Centers for Disease Control and Prevention of the United States (US CDC). Approximately 200 people in the US die each year from coccidioidomycosis [96]. Statistical data point to a major challenge for public health, with high hospital costs associated with this disease, highlighting the need for new therapeutic approaches and a vaccine against coccidioidomycosis [97]. New biological information and experimental technologies are advancing the development vaccines for Coccidioides, and NIH support for Coccidioides Collaborative Research Centers have accelerated research on this pathogen.
The pursuit of a vaccine against coccidioidomycosis ranges from the application of formalin-killed spherules to engineered mutant strains. In 1967, killed spherules were shown to protect mice against coccidioidomycosis [98]. Remarkably, vaccination with killed spherules was trialed in the US in a double-blinded human study with nearly 3,000 subjects [99]. However, the trial was limited by the toxicity of the vaccine and a low overall incidence of disease. Three decades later, soluble material from spherules was also shown to protect against lethal disease [100].
More recent studies have taken a more targeted approach to identifying specific antigens for vaccine development. Efforts have defined antigens candidates such as the protein termed antigen 2/proline-rich antigen (Ag2/Pra), immunodominant Coccidioides antigen, that resulted in the genetic vaccine with significant protection in mice [101]. The antigen Pmp1, a spherule-abundant protein, and the proteinase Cs-Ag (Coccidioides-specific antigen) were identified as immunogenic candidates [102,103]. A multivalent vaccine encapsulated with adjuvants was developed from three Coccidioides antigens (Ag2/Pra, Cs-Ag and Pmp1) resulted in the expression of the recombinant polypeptide rCpa1. Purified rCpa1 was encapsulated using four different formulations with yeast cell wall particles – mannan, chitin, glucan and mixtures with oligonucleotide (ODN). C57BL/6 and HLADR4 mice were immunized with vaccine candidates and challenged with C. posadasii infection. Both strains of mice vaccinated with GCP-rCpa1 (rCpa1 antigens encapsulated in glucan-chitin particles), had higher survival rates compared to those that received only GCPs. GCP-rCpa1 showed a significant reduction in the fungal load for HLADR4 transgenic mice when the other formulations were analyzed. Higher levels of IL-17 were also observed in mice vaccinated with GCP-rCpa1.
In a study carried out using glucan-chitin particles (GCPs), produced by the yeast Rhodotorula mucilaginosa, as an adjuvant delivery system for the Ag2 antigen examined oral delivery to improve the cell-mediated response. The results suggested that oral Ag2 delivery can contribute to protective immunity, although the best results were reached in groups that received prime and boosting subcutaneous doses. Groups that received more than one subcutaneous vaccine developed increased Th1 and Th17 responses, indicating that, for subunit vaccine development, there are potential substantial benefits to this approach [104]. Another approach for co-administration of Ag2, produced in a maize expression system (Ag2m) or bacterial culture (Ag2b), was carried out using an orally administered antigen subunit and parenteral administration of the antigen inside GCPs. To investigate the benefits of co-administration, purified and GCP-encapsulated antigens were administered to mice. The results showed a significant increase in the immune response in tissues compared to the single-way administration, favoring higher levels of response and fewer injectable vaccine doses [105].
In a cellular-vaccination approach, DCs were transfected with plasmid DNA encoding Ag2 and mice were intranasally immunized on days 0 and 10. There were no parameter differences related to lung lesions and, although no relevant cytokine responses were observed, there was an increase in specific IgG responses, indicating an immune response related to the vaccine formulation. However, further studies should be carried out to understand better the response mediated by Ag2-DCs [106]. It is noteworthy that, considering the ability of T cells activation, a DC-based approach would be especially interesting for a therapeutic vaccine [107].
The avirulent strain Δcps1, resulting from the deletion of the CPS1 gene from C. posadasii, is an important vaccine candidate, and the coccidioidomycosis models show vaccination with Δcps1 increases survival and decreases dissemination in susceptible, and immunologically normal mice [108,109]. Initial studies demonstrated that Δcps1 vaccination using various administration routes (intraperitoneal, intranasal and subcutaneous) reduces fungal burden and enhances survival in mice after virulent C. posadasii infection [108]. Further investigations with strains of mice that model primary immunodeficiency (PID) have been performed. Mice were vaccinated with arthroconidia of the strain Δcps1 or C. posadasii strain Silveira, and the animals were subsequently challenged four weeks later with the pathogenic strain C. posadasii. Vaccination with Δcps1 significantly reduces disseminated disease and the fungal burdens in the lungs and spleens compared to the control mice [110].
Δcps1 vaccine safety and efficacy have been demonstrated in dogs. Prime and booster doses of Δcps1 protect against C. posadasii strain Silveira infection in beagles, with reductions in the fungal burdens in the lungs and lymph nodes and less radiographic and histopathologic findings related to Coccidioides infection. Also, none of the dogs showed systemic reactions after vaccine administration, presenting only local swelling with spontaneous healing. Interestingly, prime-only vaccinated dogs did not develop a strong immune response, indicating that two doses are necessary for protection [111].
Preclinical vaccine analysis with another attenuated mutant Coccidioides strain, ΔT, with two chitinase genes (CTS2 and CTS3) and an upstream gene (ARD1) disrupted, has revealed important human correlate information. The ΔT strain was used to vaccinate HLA-DR4 transgenic mice, which express MHC II complex receptor that restricts the response of CD4+ T cells, followed by lethal challenge with virulent C. posadasii spores. Vaccination on HLA-DR4 mice resulted in the expansion of Th1 and Th17 cells as well as recruitment of innate inflammatory cells including macrophages, neutrophils, DCs and eosinophils into Coccidioides-infected lungs. Although vaccinated HLA-DR4 mice were susceptible to Coccidioides infection, the immunologic findings advance our understanding of vaccine biology against this complicated pathogen [112].
The research on a vaccine to combat coccidioiodomycosis is highly advanced. In particular, the data on the Δcps1 suggests that a human trail with this vaccine is appropriate.
2.6. Paracoccidioides spp.
Paracoccidioidomycosis (PCM) is a systemic disease caused mainly by two species of the genus Paracoccidioides, P. brasiliensis and P. lutzii [113]. These thermodimorphic fungi are a major threat to the health of individuals, especially in Latin America, who have significant exposures to soils or construction. In this region, PCM represents one of the most notable causes of death from chronic or recurrent parasitic and infectious diseases [114]. In experimental and human PCM, the infection causes granuloma formation through activation of Th1 type response [115,116], guiding a pathway that also triggers the activation of macrophages, which are crucial in enhancing phagocytosis and preventing the fungus spread [117]. Many elements contribute to the initiation of chronic forms, but both innate and adaptive immune responses are indispensable [118,119]. A Th1/Th17 type response results in immunoprotection of PCM, while a Th2/Th9 predominant response with T regulatory cells are associated with severe forms of the disease [119]. Furthermore, vaccines for PCM that only lower fungal load are insufficient to reduce morbidity and mortality, and a balanced cytokine response is still critical [116,118,119].
Several studies have suggested that mAbs and polyclonal antibodies (pAbs) are promising therapeutics for the treatment of PCM [116,120]. The first antibody-mediated defense against P. brasiliensis gp43 (mAb 3E) resulted in a significant reduction on fungal burden and less granuloma formation in the lungs of experimentally infected mice [121]. Antibodies impact the balance of host immune responses and, for Paracoccidioides, pAbs to acidic glycosphingolipids induce a protective immune response both in vitro and in vivo assays [122]. Interestingly, pAbs are effective in therapeutic and prophylactic models as both caused a significant reduction in fungal burdens. In the therapeutic model, IFN-γ, IL-4 and IL-12 are highlighted as specifically regulated in a protective manner. The concept of a “universal fungal vaccine” or at least one that could effectively combat multiple fungi is supported by the extensive preservation of epitopes observed within the Hsp family of dimorphic fungi, particularly H. capsulatum and Paracoccidioides [117]. In an experimental infection of P. lutzii in mice, mAbs to the Hsp60 protein from H. capsulatum (described in the Histoplasma section above) enhance P. lutzii clearance, reduce pulmonary tissue damage and contribute to a Th1-based response as well a increased phagocytosis in vitro. The combination of antibodies with antifungals is another approach for improving therapy, which is supported by a study that combined a mAb (mAb F1.4) to a cell wall glycoconjugate fraction of P. brasiliensis with antifungals that decreased fungal load and induced a Th1 and Th17 cytokine profile in an experimental murine model of PCM caused by P. brasiliensis [123]. These studies have a great impact because they can potentially be used as an alternative treatment for panfungal therapy.
DNA-based vaccines are effective against Paracoccidioides experimental infections. For example, a vaccine with peptide 10 (P10), derived from the 43-KDa glycoprotein (gp43) of P. brasiliensis, expressed in a plasmid gene (pP10) produces long lasting protective cellular immune response mediated by IFN-γ [124,125]. This vaccine reduces fungal burdens and increases the concentrations of P. brasiliensis-specific antibodies. Although gp43 can also be used as a vaccine, P10 generates a more protective immune response compared to the entire gp43 [126,127] and the peptide is much easier and cheaper to reproducibly produce. Subsequently, pP10 has been tested in a therapeutic regimen in which infected animals received the vaccine after the PCM was well established, and pP10 reduced the fungal burdens in the lungs of mice. Significantly, co-administration of plasmid murine IL-12 (pIL-12) enhances the effectiveness of pP10 [127]. Interestingly, the promoter pcDNA3 contained in the plasmid design, which is a strong stimulation of the immune system, facilitates the efficacy of the vaccine. Another study has demonstrated that combining P10 vaccination with antifungal chemotherapy can reduce treatment time and improve the cytokine profile, also exhibiting that combination therapy prevents disease relapse [128].
Despite studies showing advantages in the implementation of P10 in PCM therapy, peptides alone are, generally, poorly immunogenic and require adjuvants and delivery systems to enhance their effectiveness [129,130].
Given due consideration to this matter, a research demonstrated that P10 entrapped within Poly (lactic acid-glycolic acid) (PLGA) nanoparticles in combination with sulfamethoxazole/trimethoprim effectively increased the therapeutic effect of P10 in experimental murine PCM, and the response was improved compared to the peptide emulsified in Freund’s adjuvant [131]. Furthermore, P10 within PLGA nanoparticles reduced the amount of peptide needed for a therapeutic response, consequently reducing the cost of the treatment. Additional adjuvants combined with P10 have been tested in mice, including Alum, FliC flagellin and cationic lipids [132]. When compared to mice immunized only with P10, animals vaccinated with P10 in combination with each of these adjuvants individually had significantly improved responses resulting in only minimal detectable fungal burdens. In particular, the administration of P10 and cationic lipids, the best responding P10-adjuvant combination, significantly reduced the levels of IL-10 and increased levels of IFN-γ. Another study tested P10 peptide adsorbed onto cationic liposomes dioctadecyldimethylammonium with a trehalose dibehenate-cationic adjuvant formulation (DDA/TDB-CAF01 or just DDA/TDB). This protocol maintains the properties of P10 seen in the prior described experiments and intensifies the antifungal response in C57BL/6 mice [133].
Virus-like particles (VLP) derived from hepatitis B virus have been explored as a delivery platform for P10 [134]. The effectiveness of a diverse VLP/P10 composition in murine PCM demonstrated a single promising vaccine candidate capable of efficiently stimulating protective P10-specific CD4+ T cells. However, the VLP/P10 formulation necessitates both homologous and heterologous prime-boost regimens to evoke a persistent immune response against P. brasiliensis.
Another promising approach of a carrier system for P10 peptide is chitosan nanoparticles. Chitosan is cationic and low-cost polymer used in the formulation of structures of nanoparticles and possesses advantages, such as the ability to be mucoadhesive, which facilitates their being designed for nasal delivery of therapeutic drug payloads [135,136]. When chitosan is complexed or associated with P10 in a therapeutic protocol, the vaccine models show a mixed pattern of Th1 and Th2 type responses that reduces fungal loads. Additionally, the effective dose of chitosan nanoparticles complexed to P10 requires significantly lower amounts of P10 compared to other protocols and also results in a reduced lung tissue damage [137]. Notably, a recent study demonstrates that intranasally administered chitosan nanoparticles predominantly remain in the upper airways with a small amount localizing in the lower airways [138], which demonstrates that the immunological response is principally induced outside of the lung
DCs play important roles in stimulating adaptive responses [139] and are critical for the control of Paracoccidioides spp. infections [140–142]. Given this, investigators have examined whether DCs could be harnessed for their adjuvant-like effects for the presentation of P10 to improve the resolution of PCM. Notably, P10-primed DCs from the bone marrow (bmDCs) of mice are both protective and therapeutic in experimental PCM infection models. Subcutaneous introduction of P10-primed DCs induces a strong Th1 response with increased concentrations of IFN-γ and IL-12 with lower levels of IL-10 and IL-4, reduces the number of granulomas, mitigates damage in lung tissues, and reduces fungal burdens [143]. The effect of DCs origin has been explored and P10 priming of bmDCs or monocytes of peripheral blood (moDCs) of BALB/c mice show no significant difference in their protective capacities [144]. In vitro experiments also show that the DC-based approaches activate the superficial molecules MHC-II, CD80 and CD86 and stimulated the proliferation of T cells CD4+ and CD8+. Given the concern for PCM treatment in immunocompromised patients, P10-primed DCs treatment has also been explored in mice immunosuppressed by dexamethasone, and the DC-based therapy effectively induces specific and protective immunity against P. brasiliensis in the immunosuppressed animals [145]. Additionally, the antifungals in combination with P10-primed DCs are synergistic in lowering the fungal burden in the immunocompromised mice with PCM.
Also, there are significant differences in the amino acids sequences and gene expression patterns in the pathogenic Paracoccidioides species [146]. This is important to note in vaccine development, especially when considering platforms based on gp43, since this sequence of P. brasiliensis and P. lutzii has 81% identity between these two species, indicating that there are differences in the sequence and epitopes of this immunodominant glycoprotein [147–151]. This difference could influence the response of immunized individuals, reducing the effectiveness of the vaccine against infections from other non-P. brasiliensis species. A recent study identified peptides derived from P. lutzii through immunoprecipitation of protein components with anti-MHC-II antibodies followed by mass spectrometry and in silico predictions [119]. Three of the peptides derived from P. lutzii that were chosen based on their modeling were found to markedly stimulate the proliferation of both CD4+ and CD8+ lymphocytes and expressed a profile of superficial receptors in bmDCs that promotes effective adaptive immune responses.
The studies described for Paracoccidioides demonstrate a remarkable array of vaccine candidates for this neglected fungal pathogen. Strategies are currently being developed for trialing of a P10-based adjuvant vaccine in patients.
2.7. Sporothrix spp.
Sporotrichosis is a subcutaneous fungal infection caused by Sporothrix species related to the clinical clade: Sporothrix schenckii, S. brasiliensis, S. globosa and S. luriei [152]. S. schenckii has a global distribution and is mostly associated with sapronotic infection, in which fungi propagules are inoculated in the skin through traumatic injury due to contact with contaminated soil, decaying organic matter and plant debris. S. brasiliensis is predominant in South America, especially in Brazil, and is associated with zoonotic transmission through bites and scratches from infected cats [153,154]. The zoonotic transmission is important to the disease epidemiology, considering the rapid transmission within Brazilian States and S. brasiliensis virulent characteristics, and major efforts to improve disease prevention and control are reported in the literature [152].
There has been a robust search for immunoprotective antigens present on the Sporothrix spp cell surface with the 70 kDa glycoprotein, also known as Gp70, being one of the most studied proteins from the Sporothrix cell wall. Significantly, sera from individuals with S. schenckii recognizes 40 kDa and 70 kDa proteins extracted from S. schenckii, indicating that these proteins could be targets for therapeutic and prophylactic purposes as well as diagnosis [155]. Interestingly, the 70 kDa (Gp70) S. schenckii is homologous with the 60 kDa (Gp60) in S. brasiliensis, with molecular weight differences being due to post-translational modifications, such as glycosylation. In cats with sporotrichosis, 100% of the sera collected recognized both Gp60 and Gp70, indicating that these glycoprotein isoforms are potential vaccine candidates [156]. Furthermore, a Gp70 mAb, P6E7, was an effective experimental therapeutic vaccine formulation against S. schenckii and S. brasiliensis. However, despite a protective response over the first week after administration of the antibody, disease relapsed, and fungal burdens increased in livers and spleens at 14 and 21 days [157]. Nevertheless, increased or repeat dosing as well as combination with antifungal medications could improve the efficacy of P6E7.
An immunoproteomic approach identified a peptide, ZR8, from Gp70 and a peptide, ZR3, derived from the sequence of an impotin protein, have been assessed as potential vaccine candidates. Vaccination with either peptide reduced the sizes of S. brasiliensis lesions and enhanced Th1 and Th17 responses [158]. In a study using S. schenckii yeast cell wall proteins (ssCWP) and aluminum hydroxide (AH), vaccination with the proteins and adjuvant increased cytokine release related to Th1, Th2 and Th17 responses ex vivo, enhanced S. schenckii opsonization and reduced fungal adhesion. Passive administration of serum from mice that received the formulation with a higher dose of ssCWP (100 μg) associated with AH (AH+CWP100) reduced liver and spleen fungal burdens in animals infected with S. schenckii compared to the control group [159]. Another interesting finding was that immunoblots with sera from immunized mice showed that 47 kDa and 71 kDa proteins were recognized, indicating that there could be other potential antigens in the Sporothrix spp cell wall.
Although the vaccine formulation with AH had a good response, aluminum-based adjuvants may stimulate an inflammatory response at the vaccination site, leading to the development of inflammatory lesions in cats and, potentially, result in the develop of a sarcoma [160]. Notably, an inflammatory response was found in the AH+CWP100 formulation in mice [158]. Hence, another study evaluated Montanide™ Pet Gel A (PGA), a polymeric adjuvant, which was combined with CWP100, and the PGA+CWP100 was less toxic than the prior formulation while still stimulating a robust IgG-specific response and the sera from immunized mice enhanced phagocytosis of Sporothrix [161].
As sera from immunized mice immunized with the CWP100 recognized a 47 kDa enolase, a metalloenzyme [161], the protein was synthesized as a recombinant enolase (rSsEno) and associated with PGA Vaccination with the rSsEno and PGA resulted in a prolongation in the survival of mice infected with S. brasiliensis, which was due to an enhanced Th1/Th2 response. Administration of sera from immunized mice also reduced the fungal burdens in the spleens and livers of infected mice [162]. The vaccine was also effective against a hypervirulent S. schenckii strain, inducing a strong humoral IgG response and cellular Th1 response [163]. Interestingly, the experimental depletion of regulatory T cells (Tregs) in mutant mice increased the Th1 response after vaccination with rSsEno and PGA, indicating that T cell transient modulation could be beneficial to stimulate the immune response of interest, but further studies are needed to verify the safety of this approach [164].
Experimental Sporothrix vaccines targeting Gp70 protein and enolase demonstrate that a vaccine against sporotrichosis is possible. Given the prevalence of both S. brasiliensis and S. schenckii, future studies should prioritize candidates that will effectively prevent and/or mitigate disease caused by either species.
2.8. Pneumocystis spp.
Pneumocystis sp. is an opportunistic fungal pathogen that primarily causes pneumonia in patients with impaired immunity, which complicates vaccine development. It is also challenging that the ascus and trophozoite forms are markedly different as evidenced by variations in transcriptomic and proteomic activities. For example, the omics techniques have revealed novel form-dependent antigens (e.g., GSC-1). The ectodomain Gsc-1 is abundant in asci forms, and this has been considered as a promising target for vaccine development given that it encodes a 1,3-ß-glucan synthase. Immunization with the GSC-1 induced a strong antibody response that effectively reduced experimental Pneumocystis murina ascus burden and total fungal load, and resulted in only low-fungal burdens in uninfected co-housed mice [165].
The antigenic epitopes of the gene encoding the 55 kDa antigen fragment of Pneumocystis (p55) synthesized in the tandem antigen gene (TAG) is another potential vaccine candidate. Vaccination with a p55-TAG DNA against Pneumocystis carinii induces increases in IFN-γ and IL-17 populations and decreases the fungal burden [166]. Vaccination with the Pneumocystis cross-reactive antigen 1 (Pca1) generates antibodies that recognize Pneumocystis jirovecii and P. carinii. The Pca1 vaccine proved to be effective in both a prophylactic and a therapeutic approach [167]. Vaccination with P. carinii recombinant A121–85 antigen also generates pro-inflammatory factors in murine lungs and increases the IgG titer [168]. Vaccination with Pneumocystis Kexin 1 (KEX1) protein generates protection against Pneumocystis infections, and produces durable humoral responses and B cell memory [169]. This vaccine effectively stimulates prior immunologic memory to produce a protective response in immunocompromised mice [170]. Significantly, a KEX1-based vaccine produces protective responses in non-human primates with early chronic phase of simian immunodeficiency virus (SIV), and induces robust protective antibody titers though 28 weeks post-infection [171].
The oral vaccination strategy with live P. murina using the prime-boost immunization strategy prevents a subsequent lung infection by Pneumocystis in mice. This vaccine candidate increases the CD4+ T cells, CD8+ T cells, CD19+ B cells, and CD11b+ macrophages in the lungs infected. Serum from vaccinated mice protects mice infected with P. murina. However, intestinal microbial diversity can adversely affect oral vaccination with live P. murina [172].
2.9. Mucorales order
Mucormycosis is an opportunistic life-threatening disease caused by mucormycetes. The most commonly encountered causative agents are Rhizopus delemar (representing 70% of all cases), R. oryzae, R. azygosporus, R. stolonifer and Mucor circinelloides [173,174]. One of the unexpected lethal complications of the SARS-CoV-2 was the dramatic numbers of fungal infections in patients with complicated COVID-19. During the short period of May 5th to August 3rd, 2021, India experienced an unprecedented epidemic of COVID-19-associated murcomycosis, known as “Black fungus”. In this period, 47,508 cases and 4,425 deaths were reported due to murcomycosis in India, while 41,512 cases and 3,554 deaths additional cases worldwide occurred at the same time [175]. Despite rigorous and invasive treatment, including surgical debridement and antifungal therapy, the mortality rate of mucormycosis remains high. Although vaccine strategies for mucormycosis have historically lagged, new vaccination treatments are being developed.
A study of proteomes identified two protein candidates with similarity across several mucormycosis fungi. A pool of B- and T-cell epitopes displayed on a multi-epitope vaccine had promising immunological and physicochemical characteristics [176]. The proof-of-principle vaccine-receptor docking study, by molecular simulation and the predicted immune response, suggested that the vaccine was a potential solution against mucormycosis. Using an immunoinformatics approach, a potential polyvalent vaccine was developed that presented of the 100% conserved epitopes and targeted the key protein component responsible for iron entry, FTR1, into these species [175]. A recent study of M. circinelloides and R. oryzae proteins identified several epitopes of both fungi using population coverage and immune response analysis. A designed construct based on molecular docking analyses with two immune receptors and one natural fungal receptor that was consider as stable with only 7% disordered regions [177].
In conclusion, the COVID-19 pandemic has accelerated the search for effective mucormycosis vaccines. However, as the majority of work has been in silico to date, further rigorous in vivo studies are required to better demonstrate the utility of these platforms.
3. Conclusions
In this review, we presented some of the latest advances in the development of vaccines for preventing and/or treating several important human mycoses. Considering the perspective of the increasing numbers of cases of mycoses in the world, many of which are fatal or leave sequelae that require long and potentially toxic treatments and with the continued emergence of drug resistant clinical isolates, the development of vaccines to prevent and treat these diseases is fundamental as an alternative to control the expansion of these infectious diseases. Although several study groups are developing vaccines, especially for the mycoses highlighted by the WHO in the recent FPPL, there is still no fungal vaccine available for humans. The fact that many mycoses are opportunistic and predominantly affect immunocompromised patients further complicates the development of vaccines. It is important to also recognize that the financial incentive for the development of vaccines for a smaller target population or for geographically restricted fungi is another challenge to overcome. The ability to generate safe vaccines for commensal organisms such as Candida spp further complicates vaccine development. Despite these challenges, vaccines remain one of the best alternatives for the prevention and treatment of infectious diseases, including mycoses, especially if multi-fungal vaccines are discovered. In conclusion, the continued and highly active research on fungal vaccines suggests that the emergence of a fungal vaccine is not just a prospect, but an increasingly close reality.
4. Expert Opinion
Mortality rates linked to fungal infections have increased significantly worldwide, especially among immunosuppressed individuals. A global estimate points to more than 1.7 million deaths caused by fungal diseases, and high medical costs [4]. These data emphasize the profound impact of fungal infections and highlight the need to develop new approaches with promising results to control these infections.
Although current antifungal therapy is considered effective in many cases, the toxicity of certain antifungal medications and the emergence of treatment-resistant strains have driven the search for new therapeutic and/or preventive options. Given this scenario, several research teams are exploring immunotherapeutic strategies as a promising alternative to combat invasive fungal infections [94]. Furthermore, efforts are being focused on developing effective vaccines. Although there is not yet a vaccine available for treating or preventing fungal infections, significant progress has been made in recent years.
The difficulties encountered in obtaining a licensed vaccine against fungal infections encompass several determining factors, starting with the neglect of fungal infections. The development of safe vaccines focusing on immunocompromised individuals is also a challenge, as this population suffers most from these infections. Other obstacles lie in the similarity between fungal and mammalian cells, limiting the choice of vaccine targets. Furthermore, the lack of interest from pharmaceutical industries and attention from health agencies and policies is related to the fact that there is no vaccine against fungi available for clinical use to date. However, even with all these limitations, an increasing number of vaccine candidates have been evaluated, in addition to other immunotherapeutic options.
Epidemiological studies of fungi that are present in the environment are also extremely important, as they point out endemic regions for different species. In the future, when a vaccine is developed and reaches clinical use, people living in places with a high incidence of specific fungal pathogens and, especially immunocompromised individuals, should be prioritized in the vaccination plan.
Over the next ten years, future research exploring the combination of different methodologies used in obtaining and developing vaccines for the mycoses mentioned in this review, such as DNA vaccines, vaccine subunits, vaccines with attenuated organisms, as well as optimization and advances in the use of adjuvants, must bring satisfactory results in order to progress with new phases of investigation. Furthermore, studies involving biological knowledge, virulence, mechanisms of action and interaction of pathogenic fungi with hosts will be essential to achieve good results.
Article highlights.
Candidiasis vaccines are mostly related to C. albicans infections, with the most promising approach being the application of a recombinant Als3 antigen (NDV-3A) vaccine for recurrent vulvovaginal candidiasis, already tested in a clinical trial. NDV-3A was also tested against C. auris infection, with promising results;
Whole yeast vaccination formulations (heat-killed, genetically engineered) were evaluated for Cryptococcus neoformans and C. gattii, although most studies currently focus on subunit vaccines. The immune responses induced by these two species differ and, therefore, the ability to protect against both organisms would be ideal for a vaccine development;
Aspergillus fumigatus is a ubiquitous environmental fungus, making it difficult to find an approach that could stimulate a protective immune response since there is regular contact with the body and the propagules. Experiments testing whole fungus and extracted proteins have been assessed in murine models;
Histoplasma capsulatum has proteins commonly found in other fungi, such as Hsp60 and enolase, and these have been studied in in vivo models, with and without the use of adjuvants;
Potential vaccines against coccidioidomycosis, using distinct techniques, are in advanced development. The formulation with Δcps1, an avirulent strain, led to protection against Coccidioides posadasii in mice and also in dogs; however, human clinical studies have not been reported;
The association of Paracoccidioides brasiliensis and P. lutzii peptides, especially the peptide P10, in combination with a carrier molecule has been most rigorously explored for a vaccine against these fungi. Also, the presence of shared peptides with other fungi, such as H. capsulatum, may stimulate a pan-fungal formulation;
Subunit vaccines are currently conceptualized for Sporothrix schenckii and S. brasiliensis, using the two most immunogenic proteins previously identified, Gp70 and enolase. rSsEno, a recombinant enolase, demonstrated a good response in mice experimental infection when associated with an adjuvant;
During the COVID-19 pandemic, the incidence of mucormycosis and pneumocystosis markedly increased. Studies for Pneumocystis jirovecii vaccination are mostly related with proteins and peptides, as proteomic studies with Mucor circinelloides and Rhizopus oryzae are starting to emerge.
Acknowledgements
We would like to thank the funding agencies, listed under “Funding”, for their funding.
Funding
This work was supported by grants from the FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo; numbers: 2021/01904–7 and 2020/03607–7), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior; Code 001) and the Inova Program of Fiocruz (Fundação Oswaldo Cruz). J.D.N. and D.Z.M. were supported in part by NIH AI71093, NIH AI124797 and NIH AI165204. The funders had no role in the design of the study; in the writing of the manuscript, in the analyses, collection, interpretation of data, or in the decision to publish the results.
Footnotes
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or mending, or royalties.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- [1].World Health Organization. WHO fungal priority pathogens list to guide research, development and public health action [Internet]. Geneva. 2022. [cited 2023 Jun 18]. Available from: https://www.who.int/publications/i/item/9789240060241 [Google Scholar]
- [2].Centers for Disease Control and Prevention. Impact of Fungal Diseases in the United States [Internet]. Atlanta (GA). 2023. [cited 2023 Jun 18]. Available from: https://www.cdc.gov/fungal/cdc-and-fungal/burden.html [Google Scholar]
- [3].Gold JAW, Ahmad FB, Cisewski JA, et al. Increased Deaths From Fungal Infections During the Coronavirus Disease 2019 Pandemic—National Vital Statistics System, United States, January 2020–December 2021. Clinical Infectious Diseases. 2022;76(3):e255–e262. doi: 10.1093/cid/ciac489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kainz K, Bauer MA, Madeo F, et al. Fungal infections in humans: the silent crisis. 2020;7(6):143–145. doi: 10.15698/mic2020.06.718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhao Y, Ye L, Zhao F, et al. Cryptococcus neoformans, a global threat to human health. 2023;12(1):1–10. doi: 10.1186/s40249-023-01073-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Khalaf RA, Fattouh N, Medvecky M, et al. Whole genome sequencing of a clinical drug resistant Candida albicans isolate reveals known and novel mutations in genes involved in resistance acquisition mechanisms. J Med Microbiol. 2021;70(4):1–6. 001351. doi: 10.1099/jmm.0.001351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Armstrong-James D, Kosmidis C, Bromley M. Update on the treatment of chronic pulmonary aspergillosis. Curr Opin Infect Dis. 2023;36(2):146–151. doi: 10.1097/QCO.0000000000000913 [DOI] [PubMed] [Google Scholar]
- [8].Brilhante RSN, Guedes GM de M, da Silva MLQ, et al. A proposal for antifungal epidemiological cut-off values against Histoplasma capsulatum var. capsulatum based on the susceptibility of isolates from HIV-infected patients with disseminated histoplasmosis in Northeast Brazil. Int J Antimicrob Agents. 2018;52(2):272–277. doi: 10.1016/j.ijantimicag.2018.03.017 [DOI] [PubMed] [Google Scholar]
- [9].Baes Pereira S, Dos Reis Gomes A, Bressan Waller S, et al. Sporotrichosis in dogs: epidemiological and clinical-therapeutic profile and the emergence of itraconazole-resistant isolates. Med Mycol. 2022;60(12):1–8. doi: 10.1093/mmy/myac089 [DOI] [PubMed] [Google Scholar]
- [10].Du H, Bing J, Hu T, et al. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020;16(10):1–18. doi: 10.1371/journal.ppat.1008921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dannaoui E Antifungal resistance in mucorales. Int J Antimicrob Agents. 2017;50(5):617–621. doi: 10.1016/j.ijantimicag.2017.08.010 [DOI] [PubMed] [Google Scholar]
- [12].Nicola AM, Albuquerque P, Paes HC, et al. Antifungal drugs: New insights in research & development. Pharmacol Ther. 2019; 195:21–38. doi: 10.1016/j.pharmthera.2018.10.008 [DOI] [PubMed] [Google Scholar]
- [13].Peyclit L, Yousfi H, Rolain JM, et al. Drug Repurposing in Medical Mycology: Identification of Compounds as Potential Antifungals to Overcome the Emergence of Multidrug-Resistant Fungi. Pharmaceuticals (Basel). 2021;14(5):1–20. doi: 10.3390/ph14050488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].World Health Organization. Global Vaccine Action Plan (2011–2020) [Internet]. 2013. [cited 2023 Jun 18]. Available from: https://www.who.int/teams/immunization-vaccines-and-biologicals/strategies/global-vaccine-action-plan
- [15].Rodrigues CMC, Plotkin SA. Impact of Vaccines; Health, Economic and Social Perspectives. Front Microbiol. 2020; 14:1–15. doi: 10.3389/fmicb.2020.01526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Guinea J Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect. 2014;20 Suppl 6:5–10. doi: 10.1111/1469-0691.12539 [DOI] [PubMed] [Google Scholar]
- [17].Dadar M, Tiwari R, Karthik K, et al. Candida albicans - Biology, molecular characterization, pathogenicity, and advances in diagnosis and control - An update. Microb Pathog. 2018; 117:128–138. doi: 10.1016/j.micpath.2018.02.028 [DOI] [PubMed] [Google Scholar]
- [18].McCarty TP, White CM, Pappas PG. Candidemia and Invasive Candidiasis. Infect Dis Clin North Am. 2021;35(2):389–413. doi: 10.1016/j.idc.2021.03.007 [DOI] [PubMed] [Google Scholar]
- [19].Ahmadipour S, Field RA, Miller GJ. Prospects for anti- Candida therapy through targeting the cell wall: A mini-review. Cell Surf. 2021; 7:1–8. doi: 10.1016/j.tcsw.2021.100063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Singh S, Nabeela S, Barbarino A, et al. Antibodies targeting Candida albicans Als3 and Hyr1 antigens protect neonatal mice from candidiasis. Front Immunol. 2022; 13:1–9. doi: 10.3389/fimmu.2022.925821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Akhtar N, Singh A, Upadhyay AK, et al. Design of a multi-epitope vaccine against the pathogenic fungi Candida tropicalis using an in silico approach. Journal of Genetic Engineering & Biotechnology. 2022;20(1):1–15. doi: 10.1186/s43141-022-00415-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Akhtar N, Magdaleno JSL, Ranjan S, et al. Secreted Aspartyl Proteinases Targeted Multi-Epitope Vaccine Design for Candida dubliniensis Using Immunoinformatics. Vaccines (Basel). 2023;11(2):1–14. doi: 10.3390/vaccines11020364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Edwards JE, Schwartz MM, Schmidt CS, et al. A Fungal Immunotherapeutic Vaccine (NDV-3A) for Treatment of Recurrent Vulvovaginal Candidiasis-A Phase 2 Randomized, Double-Blind, Placebo-Controlled Trial. Clin Infect Dis. 2018;66(12):1928–1936. doi: 10.1093/cid/ciy185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Alqarihi A, Singh S, Edwards JE, et al. NDV-3A vaccination prevents C. albicans colonization of jugular vein catheters in mice. Sci Rep. 2019;9(1):1–6. doi: 10.1038/s41598-019-42517-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ibrahim AS, Luo G, Gebremariam T, et al. NDV-3 protects mice from vulvovaginal candidiasis through T- and B-cell immune response. Vaccine. 2013;31(47):5549–5556. doi: 10.1016/j.vaccine.2013.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Uppuluri P, Singh S, Alqarihi A, et al. Human Anti-Als3p Antibodies Are Surrogate Markers of NDV-3A Vaccine Efficacy Against Recurrent Vulvovaginal Candidiasis. Front Immunol. 2018;9:1–10. doi: 10.3389/fimmu.2018.01349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Akhtar N, Joshi A, Kaushik V, et al. In-silico design of a multivalent epitope-based vaccine against Candida auris. Microb Pathog. 2021; 155:1–9. doi: 10.1016/j.micpath.2021.104879 [DOI] [PubMed] [Google Scholar]
- [28].Singh S, Uppuluri P, Mamouei Z, et al. The NDV-3A vaccine protects mice from multidrug resistant Candida auris infection. PLoS Pathog. 2019;15(8):1–25. doi: 10.1371/journal.ppat.1007460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Singh S, Barbarino A, Youssef EG, et al. Protective Efficacy of Anti-Hyr1p Monoclonal Antibody against Systemic Candidiasis Due to Multi-Drug-Resistant Candida auris. J Fungi (Basel). 2023;9(1):1–16. doi: 10.3390/jof9010103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shukla M, Rohatgi S. Vaccination with Secreted Aspartyl Proteinase 2 Protein from Candida parapsilosis Can Enhance Survival of Mice during C. tropicalis-Mediated Systemic Candidiasis. Infect Immun. 2020;88(10):1–21. doi: 10.1128/IAI.00312-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gupta SK, Osmanoglu Ö, Minocha R, et al. Genome-wide scan for potential CD4+ T-cell vaccine candidates in Candida auris by exploiting reverse vaccinology and evolutionary information. Front Med (Lausanne). 2022; 9:1–14. doi: 10.3389/fmed.2022.1008527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kamli MR, Sabir JSM, Malik MA, et al. Characterization of the Secretome of Pathogenic Candida glabrata and Their Effectiveness against Systemic Candidiasis in BALB/c Mice for Vaccine Development. Pharmaceutics. 2022;14(10):1–17. Doi: 10.3390/pharmaceutics14101989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Khan T, Suleman M, Ali SS, et al. Subtractive proteomics assisted therapeutic targets mining and designing ensemble vaccine against Candida auris for immune response induction. Comput Biol Med. 2022; 145:1–12. doi: 10.1016/j.compbiomed.2022.105462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bono C, Guerrero P, Jordán-Pla A, et al. GM-CSF Programs Hematopoietic Stem and Progenitor Cells During Candida albicans Vaccination for Protection Against Reinfection. Front Immunol. 2021;12:1–17. doi: 10.3389/fimmu.2021.790309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shen H, Yu Y, Chen SM, et al. Dectin-1 Facilitates IL-18 Production for the Generation of Protective Antibodies Against Candida albicans. Front Microbiol. 2020; 11:1–16. doi: 10.3389/fmicb.2020.01648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lilly EA, Bender BE, Esher Righi S, et al. Trained Innate Immunity Induced by Vaccination with Low-Virulence Candida Species Mediates Protection against Several Forms of Fungal Sepsis via Ly6G+ Gr-1+ Leukocytes. mBio. 2021;12(5):1–13. doi: 10.1128/mBio.02548-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Martin-Cruz L, Sevilla-Ortega C, Benito-Villalvilla C, et al. A Combination of Polybacterial MV140 and Candida albicans V132 as a Potential Novel Trained Immunity-Based Vaccine for Genitourinary Tract Infections. Front Immunol. 2021;11:1–14. doi: 10.3389/fimmu.2020.612269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Martín-Cruz L, Angelina A, Baydemir I, et al. Candida albicans V132 induces trained immunity and enhances the responses triggered by the polybacterial vaccine MV140 for genitourinary tract infections. Front Immunol. 2022;13:1–13. doi: 10.3389/fimmu.2022.1066383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jin Z, Dong YT, Liu S, et al. Potential of Polyethyleneimine as an Adjuvant To Prepare Long-Term and Potent Antifungal Nanovaccine. Front Immunol. 2022; 13:1–15. doi: 10.3389/fimmu.2022.843684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Vargas G, Honorato L, Guimarães AJ, et al. Protective effect of fungal extracellular vesicles against murine candidiasis. Cell Microbiol. 2020;22(10):1–28. doi: 10.1111/cmi.13238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Xin H, Rosario-Colon JA, Eberle K. Novel Intravenous Immunoglobulin Therapy for the Prevention and Treatment of Candida auris and Candida albicans Disseminated Candidiasis. mSphere. 2023;8(1):1–12. doi: 10.1128/msphere.00584-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rajasingham R, Govender NP, Jordan A, et al. The global burden of HIV-associated cryptococcal infection in adults in 2020: a modelling analysis. Lancet Infect Dis. 2022;22(12):1748–1755. doi: 10.1016/S1473-3099(22)00499-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Devi SJN, Schneerson R, Egan W, et al. Cryptococcus neoformans serotype A glucuronoxylomannan-protein conjugate vaccines: synthesis, characterization, and immunogenicity. Infect Immun. 1991;59(10):3700–3707. doi: 10.1128/iai.59.10.3700-3707.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Devi SJN. Preclinical efficacy of a glucuronoxylomannan-tetanus toxoid conjugate vaccine of Cryptococcus neoformans in a murine model. Vaccine. 1996;14(9):841–844. doi: 10.1016/0264-410x(95)00256-z [DOI] [PubMed] [Google Scholar]
- [45].Casadevall A, Pirofski L. Insights into mechanisms of antibody-mediated immunity from studies with Cryptococcus neoformans. Curr Mol Med. 2005;5(4):421–433. doi: 10.2174/1566524054022567 [DOI] [PubMed] [Google Scholar]
- [46].Datta K, Lees A, Pirofski LA. Therapeutic Efficacy of a Conjugate Vaccine Containing a Peptide Mimotope of Cryptococcal Capsular Polysaccharide Glucuronoxylomannan. Clin Vaccine Immunol. 2008;15(8):1176–1187. doi: 10.1128/CVI.00130-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Upadhya R, Lam WC, Maybruck B, et al. Induction of Protective Immunity to Cryptococcal Infection in Mice by a Heat-Killed, Chitosan-Deficient Strain of Cryptococcus neoformans. mBio. 2016;7(3):1–14. doi: 10.1128/mBio.00547-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Colombo Ana Caroline, Rella A, Normile T, et al. Cryptococcus neoformans glucuronoxylomannan and sterylglucoside are required for host protection in an animal vaccination model. mBio. 2019;10(2):1–22. doi: 10.1128/mbio.02909-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wang Y, Wang K, Masso-Silva JA, et al. A Heat-Killed Cryptococcus Mutant Strain Induces Host Protection against Multiple Invasive Mycoses in a Murine Vaccine Model. mBio. 2019;10(6):1–18. doi: 10.1128/mBio.02145-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Rizzo J, Wong SSW, Gazi AD, et al. Cryptococcus extracellular vesicles properties and their use as vaccine platforms. J Extracell Vesicles. 2021;10(10):1–19. doi: 10.1002/jev2.12129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ueno K, Yanagihara N, Shimizu K, et al. Vaccines and Protective Immune Memory against Cryptococcosis. Biol Pharm Bull. 2020;43(2):230–239. doi: 10.1248/bpb.b19-00841 [DOI] [PubMed] [Google Scholar]
- [52].Oliveira LVN, Wang R, Specht CA, et al. Vaccines for human fungal diseases: close but still a long way to go. NPJ Vaccines. 2021;6(1):1–8. doi: 10.1038/s41541-021-00294-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Specht CA, Lee CK, Huang H, et al. Vaccination with Recombinant Cryptococcus Proteins in Glucan Particles Protects Mice against Cryptococcosis in a Manner Dependent upon Mouse Strain and Cryptococcal Species. mBio. 2017;8(6):1–14. doi: 10.1128/mBio.01872-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hester MM, Lee CK, Abraham A, et al. Protection of mice against experimental cryptococcosis using glucan particle-based vaccines containing novel recombinant antigens. Vaccine. 2020;38(3):620–626. doi: 10.1016/j.vaccine.2019.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wang R, Oliveira LVN, Lourenco D, et al. Immunological correlates of protection following vaccination with glucan particles containing Cryptococcus neoformans chitin deacetylases. NPJ Vaccines. 2023;8(1):1–11. doi: 10.1038/s41541-023-00606-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Soto ER, Specht CA, Rus F, et al. An efficient (nano) silica - In glucan particles protein encapsulation approach for improved thermal stability. J Control Release. 2023; 357:175–184. doi: 10.1016/j.jconrel.2023.03.027 [DOI] [PubMed] [Google Scholar]
- [57].Wormley FL, Perfect JR, Steele C, et al. Protection against Cryptococcosis by Using a Murine Gamma Interferon-Producing Cryptococcus neoformans Strain. Infect Immun. 2007;75(3):1453–1462. doi: 10.1128/IAI.00274-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hole CR, Wager CML, Castro-Lopez N, et al. Induction of memory-like dendritic cell responses in vivo. Nature Communications. 2019;10(1):1–13. doi: 10.1038/s41467-019-10486-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ueno K, Urai M, Sadamoto S, et al. A dendritic cell-based systemic vaccine induces long-lived lung-resident memory Th17 cells and ameliorates pulmonary mycosis. Mucosal Immunology. 2018;12(1):265–276. doi: 10.1038/s41385-018-0094-4 [DOI] [PubMed] [Google Scholar]
- [60].Zhai B, Wozniak KL, Masso-Silva J, et al. Development of protective inflammation and cell-mediated immunity against Cryptococcus neoformans after exposure to hyphal mutants. mBio. 2015;6(5):1–13. doi: 10.1128/mBio.01433-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Upadhya R, Lam WC, Hole CR, et al. Cryptococcus neoformans Cda1 and Cda2 coordinate deacetylation of chitin during infection to control fungal virulence. Cell Surf. 2021; 7:1–9. doi: 10.1016/j.tcsw.2021.100066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Specht CA, Jane Homan E, Lee CK, et al. Protection of Mice against Experimental Cryptococcosis by Synthesized Peptides Delivered in Glucan Particles. mBio. 2021;13(1):1–11. doi: 10.1128/mbio.03367-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Soto ER, Specht CA, Lee CK, et al. One Step Purification-Vaccine Delivery System. Pharmaceutics. 2023;15(5):1–11. doi: 10.3390/pharmaceutics15051390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Krüger T, Luo T, Schmidt H, et al. Challenges and Strategies for Proteome Analysis of the Interaction of Human Pathogenic Fungi with Host Immune Cells. Proteomes. 2015;3(4):467–495. doi: 10.3390/proteomes3040467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Mead ME, Knowles SL, Raja HA, et al. Characterizing the Pathogenic, Genomic, and Chemical Traits of Aspergillus fischeri, a Close Relative of the Major Human Fungal Pathogen Aspergillus fumigatus. mSphere. 2019;4(1):1–18. doi: 10.1128/mSphere.00018-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Levitz SM. Aspergillus vaccines: Hardly worth studying or worthy of hard study? Med Mycol. 2017;55(1):103–108. doi: 10.1093/mmy/myw081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Torosantucci A, Bromuro C, Chiani P, et al. A novel glyco-conjugate vaccine against fungal pathogens. J Exp Med. 2005;202(5):597–606. doi: 10.1084/jem.20050749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Rayens E, Rabacal W, Willems HME, et al. Immunogenicity and protective efficacy of a pan-fungal vaccine in preclinical models of aspergillosis, candidiasis, and pneumocystosis. PNAS nexus. 2022;1(5):1–15. doi: 10.1093/pnasnexus/pgac248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Cenci E, Mencacci A, Bacci A, et al. T cell vaccination in mice with invasive pulmonary aspergillosis. J Immunol. 2000;165(1):381–388. doi: 10.4049/jimmunol.165.1.381 [DOI] [PubMed] [Google Scholar]
- [70].Namvar S, Warn P, Farnell E, et al. Aspergillus fumigatus proteases, Asp f 5 and Asp f 13, are essential for airway inflammation and remodelling in a murine inhalation model. Clin Exp Allergy. 2015;45(5):982–993. doi: 10.1111/cea.12426 [DOI] [PubMed] [Google Scholar]
- [71].Rivera A, Van Epps HL, Hohl TM, et al. Distinct CD4+-T-cell responses to live and heat-inactivated Aspergillus fumigatus conidia. Infect Immun. 2005;73(11):7170–7179. doi: 10.1128/IAI.73.11.7170-7179.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Clemons KV, Martinez M, Chen V, et al. Protection against experimental aspergillosis by heat-killed yeast is not antibody dependent. Med Mycol. 2014;52(4):422–426. doi: 10.1093/mmy/myt015 [DOI] [PubMed] [Google Scholar]
- [73].Fernandes CM, Normile TG, Fabri JHTM, et al. Vaccination with Live or Heat-Killed Aspergillus fumigatus Δ sglA Conidia Fully Protects Immunocompromised Mice from Invasive Aspergillosis. mBio. 2022;13(5):1–19. doi: 10.1128/mbio.02328-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kniemeyer O, Ebel F, Krüger T, et al. Immunoproteomics of Aspergillus for the development of biomarkers and immunotherapies. Proteomics Clin Appl. 2016;10(9–10):910–921. doi: 10.1002/prca.201600053 [DOI] [PubMed] [Google Scholar]
- [75].Levitz SM, Huang H, Ostroff GR, et al. Exploiting fungal cell wall components in vaccines. Semin Immunopathol. 2015;37(2):199–207. doi: 10.1007/s00281-014-0460-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Slarve M, Holznecht N, Reza H, et al. Recombinant Aspergillus fumigatus antigens Asp f 3 and Asp f 9 in liposomal vaccine protect mice against invasive pulmonary aspergillosis. Vaccine. 2022;40(31):4160–4168. doi: 10.1016/j.vaccine.2022.05.057 [DOI] [PubMed] [Google Scholar]
- [77].Chaturvedi AK, Kavishwar A, Keshava GBS, et al. Monoclonal immunoglobulin G1 directed against Aspergillus fumigatus cell wall glycoprotein protects against experimental murine aspergillosis. Clin Diagn Lab Immunol. 2005;12(9):1063–1068. doi: 10.1128/CDLI.12.9.1063-1068.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Eraso IC, Sangiovanni S, Morales EI, et al. Use of monoclonal antibodies for allergic bronchopulmonary aspergillosis in patients with asthma and cystic fibrosis: literature review. Ther Adv Respir Dis. 2020; 14:1–16. doi: 10.1177/1753466620961648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Ansari S, Mousavi A, Safarnejad MR, et al. Selection and characterization of two monoclonal antibodies specific for the Aspergillus flavus major antigenic cell wall protein Aflmp1. Fungal Biol. 2021;125(8):621–629. doi: 10.1016/j.funbio.2021.03.004 [DOI] [PubMed] [Google Scholar]
- [80].Bozza S, Perruccio K, Montagnoli C, et al. A dendritic cell vaccine against invasive aspergillosis in allogeneic hematopoietic transplantation. Blood. 2003;102(10):3807–3814. doi: 10.1182/blood-2003-03-0748 [DOI] [PubMed] [Google Scholar]
- [81].Perruccio K, Tosti A, Burchielli E, et al. Transferring functional immune responses to pathogens after haploidentical hematopoietic transplantation. Blood. 2005;106(13):4397–4406. doi: 10.1182/blood-2005-05-1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wang R, Wan Z, Li R. Th and Treg response induced by Aspergillus fumigatus pulsed dendritic cells in vitro. Chin Med J (Engl). 2014;127(20):3616–3622. [PubMed] [Google Scholar]
- [83].Pattison HT, Millar BC, Moore JE. Fungal vaccines. Br J Biomed Sci. 2021;78(4):167–176. doi: 10.1080/09674845.2021.1907953 [DOI] [PubMed] [Google Scholar]
- [84].Da Silva LBR, Taborda CP, Nosanchuk JD. Advances in Fungal Peptide Vaccines. Journal of Fungi. 2020;6(3):1–18. doi: 10.3390/jof6030119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Deepe GS, Buesing WR, Ostroff GR, et al. Vaccination with an alkaline extract of Histoplasma capsulatum packaged in glucan particles confers protective immunity in mice. Vaccine. 2018;36(23):3359–3367. doi: 10.1016/j.vaccine.2018.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Araúz AB, Papineni P. Histoplasmosis. Infect Dis Clin North Am. 2021;35(2):471–491. doi: 10.1016/j.idc.2021.03.011 [DOI] [PubMed] [Google Scholar]
- [87].Gomez AM, Rhodes JC, Deepe GS. Antigenicity and Immunogenicity of an Extract from the Cell Wall and Cell Membrane of Histoplasma capsulatum Yeast Cells. Infect Immun. 1991;59(1):330–336. doi: 10.1128/iai.59.1.330-336.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Roth MT, Zamith-Miranda D, Nosanchuk JD. Immunization Strategies for the Control of Histoplasmosis. Curr Trop Med Rep. 2019;6(2):35–41. doi: 10.1007/s40475-019-00172-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Deepe GS, Gibbons RS. Cellular and molecular regulation of vaccination with heat shock protein 60 from Histoplasma capsulatum. Infect Immun. 2002;70(7):3759–3767. doi: 10.1128/IAI.70.7.3759-3767.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Deepe GS. Preventive and therapeutic vaccines for fungal infections: From concept to implementation. Expert Rev Vaccines. 2004;3(6):701–709. doi: 10.1586/14760584.3.6.701 [DOI] [PubMed] [Google Scholar]
- [91].Guimaraes AJ, Frases S, Gomez FJ, et al. Monoclonal antibodies to heat shock protein 60 alter the pathogenesis of histoplasma capsulatum. Infect Immun. 2009;77(4):1357–1367. doi : 10.1128/IAI.01443-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Nosanchuk JD, Zancopé-Oliveira RM, Hamilton AJ, et al. Antibody therapy for histoplasmosis. Front Microbiol. 2012; 3:1–7. doi: 10.3389/fmicb.2012.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Deepe GS, Durose GG. Immunobiological Activity of Recombinant H Antigen from Histoplasma capsulatum. Infect Immun. 1995;63(8):3151–3157. doi: 10.1128/iai.63.8.3151-3157.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kischkel B, Boniche-Alfaro C, Menezes I de G, et al. Immunoproteomic and Immunopeptidomic Analyses of Histoplasma capsulatum Reveal Promiscuous and Conserved Epitopes Among Fungi With Vaccine Potential. Front Immunol. 2021;12:1–20. doi: 10.3389/fimmu.2021.764501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Almeida PCS, Roque BS, Felice AG, et al. Comparative Genomics of Histoplasma capsulatum and Prediction of New Vaccines and Drug Targets. J Fungi (Basel). 2023;9(2):1–14. doi: 10.3390/jof9020193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Centers for Disease Control and Prevention. Valley Fever (Coccidioidomycosis) Statistics [Internet]. USA. 2022. [cited 2023 Jun 23]. Available from: World Health Organization. WHO fungal priority pathogens list to guide research, development and public health action [Internet]. Geneva. 2022 [cited 2023 Jun 18]. Available from: https://www.cdc.gov/fungal/diseases/coccidioidomycosis/statistics.html#:~:text=On%20average%2C%20there%20were%20approximately,Multiple%20Cause%20of%20Death%20data. [Google Scholar]
- [97].Sondermeyer GL, Lee LA, Gilliss D, et al. Coccidioidomycosis-Associated Deaths in California, 2000–2013. 2016;131(4):531–535. doi: 10.1177/0033354916662210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Kong YC, Levine HB. Experimentally induced immunity in the mycoses. Bacteriol Rev. 1967;31(1):35–53. doi: 10.1128/br.31.1.35-53.1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Pappagianis D Evaluation of the protective efficacy of the killed Coccidioides immitis spherule vaccine in humans. The Valley Fever Vaccine Study Group. Am Rev Respir Dis. 1993;148(3):656–660. doi: 10.1164/ajrccm/148.3.656 [DOI] [PubMed] [Google Scholar]
- [100].Zimmermann CR, Johnson SM, Martens GW, et al. Protection against lethal murine coccidioidomycosis by a soluble vaccine from spherules. Infect Immun. 1998;66(5):2342–2345. doi: 10.1128/IAI.66.5.2342-2345.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Cox RA, Magee DM. Coccidioidomycosis: Host Response and Vaccine Development. Clin Microbiol Rev. 2004;17(4):804–839. doi: 10.1128/CMR.17.4.804-839.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Orsborn KI, Shubitz LF, Peng T, et al. Protein Expression Profiling of Coccidioides posadasii by Two-Dimensional Differential In-Gel Electrophoresis and Evaluation of a Newly Recognized Peroxisomal Matrix Protein as a Recombinant Vaccine Candidate. Infect Immun. 2006;74(3):1865–1872. doi: 10.1128/IAI.74.3.1865-1872.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Pan S, Cole GT. Molecular and biochemical characterization of a Coccidioides immitis-specific antigen. Infect Immun. 1995;63(10):3994–4002. doi: 10.1128/iai.63.10.3994-4002.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Hayden CA, Hung CY, Zhang H, et al. Maize-Produced Ag2 as a Subunit Vaccine for Valley Fever. J Infect Dis. 2019;220(4):615–623. doi: 10.1093/infdis/jiz196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Hayden CA, Landrock D, Hung CY, et al. Co-Administration of Injected and Oral Vaccine Candidates Elicits Improved Immune Responses over Either Route Alone. Vaccines (Basel). 2020;8(1):1–13. doi: 10.3390/vaccines8010037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Awasthi S, Vilekar P, Conkleton A, et al. Dendritic cell-based immunization induces Coccidioides Ag2/PRA-specific immune response. Vaccine. 2019;37(12):1685–1691. doi: 10.1016/j.vaccine.2019.01.034 [DOI] [PubMed] [Google Scholar]
- [107].Castro-Lopez N, Hung CY. Immune Response to Coccidioidomycosis and the Development of a Vaccine. Microorganisms. 2017;5(1):1–14. doi: 10.3390/microorganisms5010013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Narra HP, Shubitz LF, Mandel MA, et al. A Coccidioides posadasii CPS1 Deletion Mutant Is Avirulent and Protects Mice from Lethal Infection. Infect Immun. 2016;84(10):3007–3016. doi: 10.1128/IAI.00633-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Shubitz LF, Powell DA, Trinh HT, et al. Viable spores of Coccidioides posadasii Δcps1 are required for vaccination and provide long lasting immunity. Vaccine. 2018;36(23):3375–3380. doi: 10.1016/j.vaccine.2018.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Powell DA, Hsu AP, Butkiewicz CD, et al. Vaccine Protection of Mice With Primary Immunodeficiencies Against Disseminated Coccidioidomycosis. Front Cell Infect Microbiol. 2022; 11:1–6. doi: 10.3389/fcimb.2021.790488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Shubitz LF, Robb EJ, Powell DA, et al. Δcps1 vaccine protects dogs against experimentally induced coccidioidomycosis. Vaccine. 2021;39(47):6894–6901. doi: 10.1016/j.vaccine.2021.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Hurtgen BJ, Castro-Lopez N, Jiménez-Alzate M del P, et al. Preclinical identification of vaccine induced protective correlates in human leukocyte antigen expressing transgenic mice infected with Coccidioides posadasii. Vaccine. 2016;34(44):5336–5343. doi: 10.1016/j.vaccine.2016.08.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Martinez R New Trends in Paracoccidioidomycosis Epidemiology. J Fungi (Basel). 2017;3(1):1–13. doi: 10.3390/jof3010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Prado M, da Silva MB, Laurenti R, et al. Mortality due to systemic mycoses as a primary cause of death or in association with AIDS in Brazil: a review from 1996 to 2006. Mem Inst Oswaldo Cruz. 2009;104(3):513–521. doi: 10.1590/s0074-02762009000300019 [DOI] [PubMed] [Google Scholar]
- [115].Shikanai-Yasuda MA, Mendes RP, Colombo AL, et al. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Rev Soc Bras Med Trop. 2017;50(5):715–740. doi: 10.1590/0037-8682-0230-2017 [DOI] [PubMed] [Google Scholar]
- [116].De Mattos Grosso D, De Almeida SR, Mariano M, et al. Characterization of gp70 and anti-gp70 monoclonal antibodies in Paracoccidioides brasiliensis pathogenesis. Infect Immun. 2003;71(11):6534–6542. doi: 10.1128/IAI.71.11.6534-6542.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Thomaz L, Nosanchuk JD, Rossi DCP, et al. Monoclonal antibodies to heat shock protein 60 induce a protective immune response against experimental Paracoccidioides lutzii. Microbes Infect. 2014;16(9):788–795. doi: 10.1016/j.micinf.2014.08.004 [DOI] [PubMed] [Google Scholar]
- [118].Travassos LR, Taborda CP. Linear Epitopes of Paracoccidioides brasiliensis and Other Fungal Agents of Human Systemic Mycoses As Vaccine Candidates. Front Immunol. 2017;8:1–11. doi: 10.3389/fimmu.2017.00224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Silva LBR, Taira CL, Cleare LG, et al. Identification of Potentially Therapeutic Immunogenic Peptides From Paracoccidioides lutzii Species. Front Immunol. 2021;12:1–14. doi: 10.3389/fimmu.2021.670992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Boniche C, Rossi SA, Kischkel B, et al. Immunotherapy against Systemic Fungal Infections Based on Monoclonal Antibodies. J Fungi (Basel). 2020;6(1):1–28. doi: 10.3390/jof6010031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Buissa-Filho R, Puccia R, Marques AF, et al. “The monoclonal antibody against the major diagnostic antigen of Paracoccidioides brasiliensis mediates immune protection in infected BALB/c mice challenged intratracheally with the fungus.” Infect Immun. 2008;76(7): 3321–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Bueno RA, Thomaz L, Muñoz JE, et al. Antibodies Against Glycolipids Enhance Antifungal Activity of Macrophages and Reduce Fungal Burden After Infection with Paracoccidioides brasiliensis. Front Microbiol. 2016;7:1–10. doi: 10.3389/fmicb.2016.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Boniche-Alfaro C, Kischkel B, Thomaz L, et al. Antibody- Based Immunotherapy Combined With Antimycotic Drug TMP- SMX to Treat Infection With Paracoccidioides brasiliensis. Front Immunol. 2021; 12:1–12. doi: 10.3389/fimmu.2021.725882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Braga CJM, Rittner GMG, Henao JEM, et al. Paracoccidioides brasiliensis Vaccine Formulations Based on the gp43-Derived P10 Sequence and the Salmonella enterica FliC Flagellin. Infect Immun. 2009;77(4):1700–1707. doi: 10.1128/IAI.01470-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].de Amorim J, Magalhães A, Muñoz JE, et al. DNA vaccine encoding peptide P10 against experimental paracoccidioidomycosis induces long-term protection in presence of regulatory T cells. Microbes Infect. 2013;15(3):181–191. doi: 10.1016/j.micinf.2012.11.007 [DOI] [PubMed] [Google Scholar]
- [126].Taborda CP, Juliano MA, Puccia R, et al. Mapping of the T-cell epitope in the major 43-kilodalton glycoprotein of Paracoccidioides brasiliensis which induces a Th-1 response protective against fungal infection in BALB/c mice. Infect Immun. 1998;66(2):786–793. doi: 10.1128/IAI.66.2.786-793.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Rittner GMG, Muñoz JE, Marques AF, et al. Therapeutic DNA vaccine encoding peptide P10 against experimental paracoccidioidomycosis. PLoS Negl Trop Dis. 2012;6(2):1–9. doi: 10.1371/journal.pntd.0001519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Marques AF, Da Silva MB, Juliano MAP, et al. Peptide immunization as an adjuvant to chemotherapy in mice challenged intratracheally with virulent yeast cells of Paracoccidioides brasiliensis. Antimicrob Agents Chemother. 2006;50(8):2814–2819. doi: 10.1128/AAC.00220-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Li W, Joshi MD, Singhania S, et al. Peptide Vaccine: Progress and Challenges. Vaccines (Basel). 2014;2(3):515–536. doi: 10.3390/vaccines203051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Malonis RJ, Lai JR, Vergnolle O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem Rev. 2020;120(6):3210–3229. doi: 10.1021/acs.chemrev.9b00472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Amaral AC, Marques AF, Muñoz JE, et al. Poly (lactic acid-glycolic acid) nanoparticles markedly improve immunological protection provided by peptide P10 against murine paracoccidioidomycosis. Br J Pharmacol. 2010;159(5):1126–1132. doi: 10.1111/j.1476-5381.2009.00617.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Mayorga O, Muñoz JE, Lincopan N, et al. The role of adjuvants in therapeutic protection against paracoccidioidomycosis after immunization with the P10 peptide. Front Microbiol. 2012; 3:1–6. doi: 10.3389/fmicb.2012.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].de Araújo MV, Dos Santos Júnior SR, Nosanchuk JD, et al. Therapeutic Vaccination with Cationic Liposomes Formulated with Dioctadecyldimethylammonium and Trehalose Dibehenate (CAF01) and Peptide P10 Is Protective in Mice Infected with Paracoccidioides brasiliensis. J Fungi (Basel). 2020;6(4):1–16. doi: 10.3390/jof6040347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Holanda RA, Muñoz JE, Dias LS, et al. Recombinant vaccines of a CD4+ T-cell epitope promote efficient control of Paracoccidioides brasiliensis burden by restraining primary organ infection. PLoS Negl Trop Dis. 2017;11(9):1–20. doi: 10.1371/journal.pntd.0005927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Casettari L, Illum L. Chitosan in nasal delivery systems for therapeutic drugs. J Control Release. 2014; 190:189–200. doi: 10.1016/j.jconrel.2014.05.003 [DOI] [PubMed] [Google Scholar]
- [136].Garg A, Dewangan HK. Nanoparticles as Adjuvants in Vaccine Delivery. Crit Rev Ther Drug Carrier Syst. 2020;37(2):183–204. doi: 10.1615/CritRevTherDrugCarrierSyst.2020033273 [DOI] [PubMed] [Google Scholar]
- [137].Dos Santos Junior SR, da Silva FKL, Dias LS, et al. Intranasal Vaccine Using P10 Peptide Complexed within Chitosan Polymeric Nanoparticles as Experimental Therapy for Paracoccidioidomycosis in Murine Model. J Fungi (Basel). 2020;6(3):1–14. doi: 10.3390/jof6030160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Dos Santos Júnior SR, Barbalho FV, Nosanchuk JD, et al. Biodistribution and Adjuvant Effect of an Intranasal Vaccine Based on Chitosan Nanoparticles against Paracoccidioidomycosis. J Fungi (Basel). 2023;9(2):1–18. doi: 10.3390/jof9020245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Eisenbarth SC. Dendritic cell subsets in T cell programming: location dictates function. Nat Rev Immunol. 2019;19(2):89–103. doi: 10.1038/s41577-018-0088-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Ferreira KS, Bastos KR, Russo M, et al. Interaction between Paracoccidioides brasiliensis and pulmonary dendritic cells induces interleukin-10 production and toll-like receptor-2 expression: possible mechanisms of susceptibility. J Infect Dis. 2007;196(7):1108–1115. doi: 10.1086/521369 [DOI] [PubMed] [Google Scholar]
- [141].Travassos LR, Taborda CP. New advances in the development of a vaccine against paracoccidioidomycosis. Front Microbiol. 2012; 3:1–6. doi: 10.3389/fmicb.2012.00212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Jannuzzi GP, de Almeida JRF, dos Santos SS, et al. Notch Signaling is Required for Dendritic Cell Maturation and T Cell Expansion in Paracoccidioidomycosis. Mycopathologia. 2018;183(5):739–749. doi: 10.1007/s11046-018-0276-3 [DOI] [PubMed] [Google Scholar]
- [143].Magalhães A, Ferreira KS, Almeida SR, et al. Prophylactic and therapeutic vaccination using dendritic cells primed with peptide 10 derived from the 43-kilodalton glycoprotein of Paracoccidioides brasiliensis. Clin Vaccine Immunol. 2012;19(1):23–29. doi: 10.1128/CVI.05414-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Silva LBR, Taira CL, Dias LS, et al. Experimental therapy of Paracoccidioidomycosis using p10-primed monocyte-derived dendritic cells isolated from infected mice. Front Microbiol. 2019;10:1–12. doi: 10.3389/fmicb.2019.01727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Silva LBR, Dias LS, Rittner GMG, et al. Dendritic Cells Primed with Paracoccidioides brasiliensis Peptide P10 Are Therapeutic in Immunosuppressed Mice with Paracoccidioidomycosis. Front Microbiol. 2017;8:1–10. doi: 10.3389/fmicb.2017.01057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Puccia R, McEwen JG, Cisalpino PS. Diversity in Paracoccidioides brasiliensis. The PbGP43 gene as a genetic marker. Mycopathologia. 2008;165(4–5):275–287. doi: 10.1007/s11046-007-9055-2 [DOI] [PubMed] [Google Scholar]
- [147].Rodrigues AM, Kubitschek-Barreira PH, Pinheiro BG, et al. Immunoproteomic Analysis Reveals Novel Candidate Antigens for the Diagnosis of Paracoccidioidomycosis Due to Paracoccidioides lutzii. J Fungi (Basel). 2020;6(4):1–36. doi: 10.3390/jof6040357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Leitão NP, Vallejo MC, Conceição PM, et al. Paracoccidioides lutzii Plp43 is an active glucanase with partial antigenic identity with P. brasiliensis gp43. PLoS Negl Trop Dis. 2014;8(8):1–9. doi: 10.1371/journal.pntd.0003111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Matute DR, McEwen JG, Puccia R, et al. Cryptic speciation and recombination in the fungus Paracoccidioides brasiliensis as revealed by gene genealogies. Mol Biol Evol. 2006;23(1):65–73. doi: 0.1093/molbev/msj008 [DOI] [PubMed] [Google Scholar]
- [150].De Almeida JN, Del Negro GMB, Grenfell RC, et al. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for differentiation of the dimorphic fungal species Paracoccidioides brasiliensis and Paracoccidioides lutzii. J Clin Microbiol. 2015;53(4):1383–1386. doi: 10.1128/JCM.02847-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].de Oliveira LBZ, Della Coletta AM, Gardizani TP, et al. Molecular Phylogenetic Analysis of Paracoccidioides Species Complex Present in Paracoccidioidomycosis Patient Tissue Samples. Microorganisms. 2023;11(3):1–13. doi: 10.3390/microorganisms11030562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Rodrigues AM, Gonçalves SS, de Carvalho JA, et al. Current Progress on Epidemiology, Diagnosis, and Treatment of Sporotrichosis and Their Future Trends. J Fungi (Basel). 2022;8(8):1–32. doi: 10.3390/jof8080776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Rodrigues AM, Sybren De Hoog G, Pires De Camargo Z. Sporothrix Species Causing Outbreaks in Animals and Humans Driven by Animal-Animal Transmission The Past and Present of Sporothrix. 2016;12(7):1–7. doi: 10.1371/journal.ppat.1005638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Zhang Y, Hagen F, Stielow B, et al. Phylogeography and evolutionary patterns in Sporothrix spanning more than 14 000 human and animal case reports. Persoonia. 2015; 35:1–20. doi: 10.3767/003158515X687416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Scott EN, Muchmore HG. Immunoblot analysis of antibody responses to Sporothrix schenckii. J Clin Microbiol. 1989;27(2):300–304. doi: 10.1128/jcm.27.2.300-304.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Rodrigues AM, Fernandes GF, Araujo LM, et al. Proteomics-Based Characterization of the Humoral Immune Response in Sporotrichosis: Toward Discovery of Potential Diagnostic and Vaccine Antigens. PLoS Negl Trop Dis. 2015;9(8):1–18. doi: 10.1371/journal.pntd.0004016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].De Almeida JRF, Kaihami GH, Jannuzzi GP, et al. Therapeutic vaccine using a monoclonal antibody against a 70-kDa glycoprotein in mice infected with highly virulent Sporothrix schenckii and Sporothrix brasiliensis. Med Mycol. 2015;53(1):42–50. doi: 10.1093/mmy/myu049 [DOI] [PubMed] [Google Scholar]
- [158].De Almeida JRF, Jannuzzi GP, Kaihami GH, et al. An immunoproteomic approach revealing peptides from Sporothrix brasiliensis that induce a cellular immune response in subcutaneous sporotrichosis. Sci Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-22709-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Portuondo DL, Batista-Duharte A, Ferreira LS, et al. A cell wall protein-based vaccine candidate induce protective immune response against Sporothrix schenckii infection. Immunobiology. 2016;221(2):300–309. doi: 10.1016/j.imbio.2015.10.005 [DOI] [PubMed] [Google Scholar]
- [160].Saba C Vaccine-associated feline sarcoma: current perspectives. Vet Med (Auckl). 2017; 8:13–20. doi: 10.2147/VMRR.S116556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Portuondo DL, Batista-Duharte A, Ferreira LS, et al. Comparative efficacy and toxicity of two vaccine candidates against Sporothrix schenckii using either Montanide™ Pet Gel A or aluminum hydroxide adjuvants in mice. Vaccine. 2017;35(34):4430–4436. doi: 10.1016/j.vaccine.2017.05.046 [DOI] [PubMed] [Google Scholar]
- [162].Portuondo DL, Dores-Silva PR, Ferreira LS, et al. Immunization with recombinant enolase of Sporothrix spp. (rSsEno) confers effective protection against sporotrichosis in mice. Sci Rep. 2019;9(1):1–14. doi: 10.1038/s41598-019-53135-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Téllez-Martínez D, Portuondo DL, Loesch ML, et al. A Recombinant Enolase-MontanideTM PetGel A Vaccine Promotes a Protective Th1 Immune Response against a Highly Virulent Sporothrix schenckii by Toluene Exposure. Pharmaceutics. 2019;11(3):1–13. doi: 10.3390/pharmaceutics11030144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Batista-Duharte A, Téllez-Martínez D, Portuondo DL, et al. Selective depletion of regulatory T cells enhances the immunogenicity of a recombinant-based vaccine against Sporothrix spp. Front Cell Infect Microbiol. 2023;12:1–9. doi: 10.3389/fcimb.2022.1084526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Eddens T, Elsegeiny W, Ricks D, et al. Transcriptomic and Proteomic Approaches to Finding Novel Diagnostic and Immunogenic Candidates in Pneumocystis. mSphere. 2019;4(5):1–13. doi: 10.1128/mSphere.00488-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Fan H, Guo JY, Ma SL, et al. Synthetic p55 tandem DNA vaccine against Pneumocystis carinii in rats. Microbiol Immunol. 2016;60(6):397–406. doi: 10.1111/1348-0421.12386 [DOI] [PubMed] [Google Scholar]
- [167].Tesini BL, Wright TW, Malone JE, et al. Immunization with Pneumocystis Cross-Reactive Antigen 1 (Pca1) Protects Mice against Pneumocystis Pneumonia and Generates Antibody to Pneumocystis jirovecii. Infect Immun. 2017;85(4):1–8. doi: 10.1128/IAI.00850-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Tong T, Wang Z, Xu Y, et al. Immunization with Pneumocystis carinii A121–85 antigen activates immune function against P. carinii. BMC Immunol. 2021;22(1):1–8. doi: 10.1186/s12865-021-00436-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Cobos Jiménez V, Rabacal W, Rayens E, et al. Immunization with Pneumocystis recombinant KEX1 induces robust and durable humoral responses in immunocompromised non-human primates. Hum Vaccin Immunother. 2019;15(9):2075–2080. doi: 10.1080/21645515.2019.1631135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Kling HM, Norris KA. Vaccine-Induced Immunogenicity and Protection Against Pneumocystis Pneumonia in a Nonhuman Primate Model of HIV and Pneumocystis Coinfection. J Infect Dis. 2016;213(10):1586–1595. doi: 10.1093/infdis/jiw032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Rabacal W, Schweitzer F, Kling HM, et al. A therapeutic vaccine strategy to prevent Pneumocystis pneumonia in an immunocompromised host in a non-human primate model of HIV and Pneumocystis co-infection. Front Immunol. 2022;13:1–13. doi: 10.3389/fimmu.2022.1036658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Samuelson DR, de la Rua NM, Charles TP, et al. Oral Immunization of Mice with Live Pneumocystis murina Protects against Pneumocystis Pneumonia. The Journal of Immunology. 2016;196(6):2655–2665. doi: 10.4049/jimmunol.1502004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556–569. doi: 10.1128/CMR.18.3.556-569.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Tabassum T, Araf Y, Moin AT, et al. COVID-19-associated-mucormycosis: possible role of free iron uptake and immunosuppression. Mol Biol Rep. 2022;49(1):747–754. doi: 10.1007/s11033-021-06862-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Araf Y, Moin AT, Timofeev VI, et al. Immunoinformatic Design of a Multivalent Peptide Vaccine Against Mucormycosis: Targeting FTR1 Protein of Major Causative Fungi. Front Immunol. 2022; 13:1–16. doi: 10.3389/fimmu.2022.863234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Soltan MA, Eldeen MA, Elbassiouny N, et al. In Silico Designing of a Multitope Vaccine against Rhizopus microsporus with Potential Activity against Other Mucormycosis Causing Fungi. Cells. 2021;10(11):1–25. doi: 10.3390/cells10113014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Naveed M, Ali U, Karobari MI, et al. A Vaccine Construction against COVID-19-Associated Mucormycosis Contrived with Immunoinformatics-Based Scavenging of Potential Mucoralean Epitopes. Vaccines (Basel). 2022;10(5):1–22. doi: 10.3390/vaccines10050664 [DOI] [PMC free article] [PubMed] [Google Scholar]


