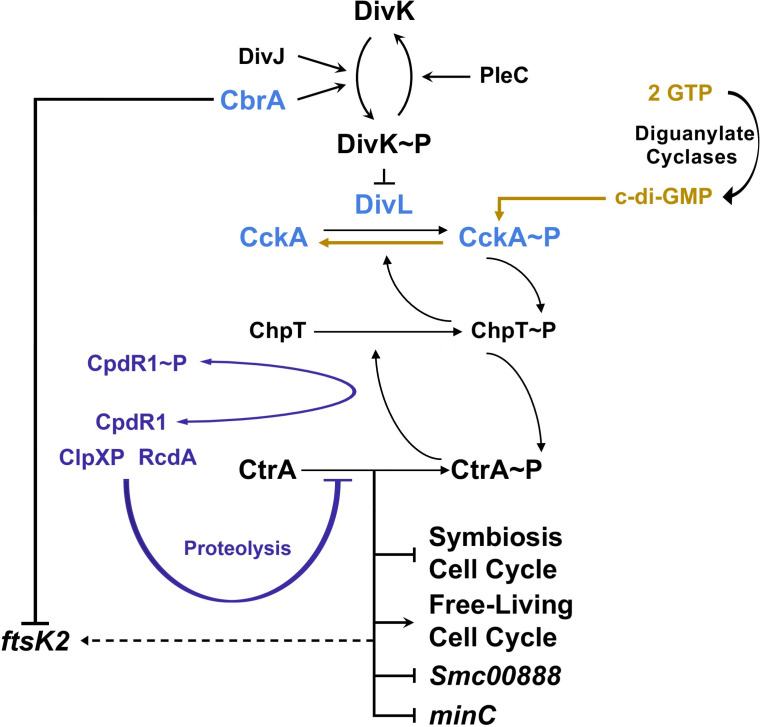
Fig 1.
Model for CtrA-dependent cell cycle regulation in S. meliloti. DivJ and CbrA have been shown to function as DivK kinases, while PleC is a DivK phosphatase (11, 15, 16). When phosphorylated, DivK~P likely inhibits the ability of DivL to bind CckA and activate its kinase activity, thereby leading to the loss of CtrA~P activity. This TCS pathway regulating CtrA phosphorylation status is required to couple DNA replication initiation with cell division during free-living reproduction as well as bacteroid cell cycle differentiation during symbiosis (15, 16). CpdR1 is known to promote the proteolysis of CtrA via RcdA (highlighted in purple) (13, 14, 17). In addition, CckA phosphatase activity is enhanced in the presence of high concentrations of c-di-GMP, and this likely reduces the activity of CtrA downstream (highlighted in gold, this study). At present, the identification of divL and cckA symbiosis suppressors of ΔcbrA strongly supports the model that DivL and CckA act as a kinase/phosphate switch in conjunction with DivK to control the downstream phosphorylation status of CtrA (highlighted in blue, this study) through the histidine phosphotransferase ChpT. More specifically, the CckAA373S suppressor protein is defective for kinase activity (this study), and it is, therefore, likely that repression of CtrA activity is critical to allowing symbiotic cell cycle differentiation. Downstream transcriptional changes to cell cycle and CtrA-regulated genes Smc00888 and minC may contribute to the modulation of c-di-GMP levels and the process of cell division, respectively (this study). While CtrA indirectly affects the expression of ftsk2 (14), and this cell cycle-regulated gene is transcriptionally repressed by CbrA in a manner independent of CtrA activity (this study).