Abstract
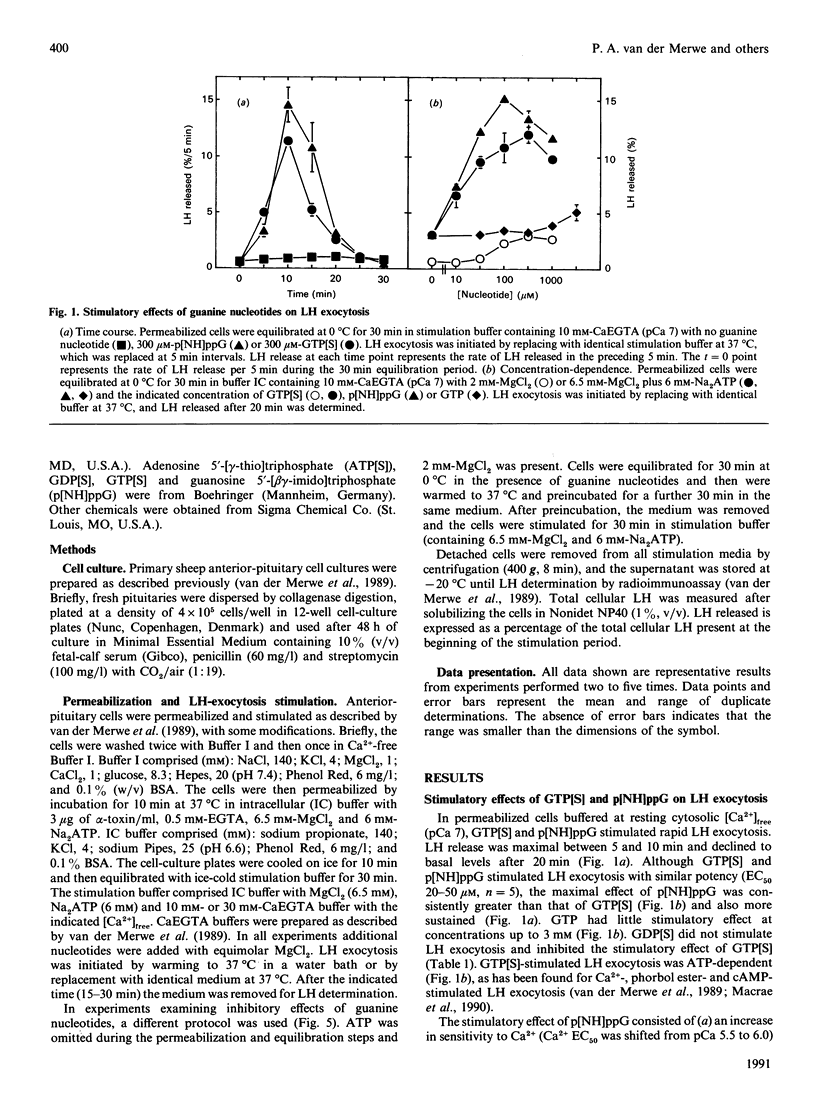
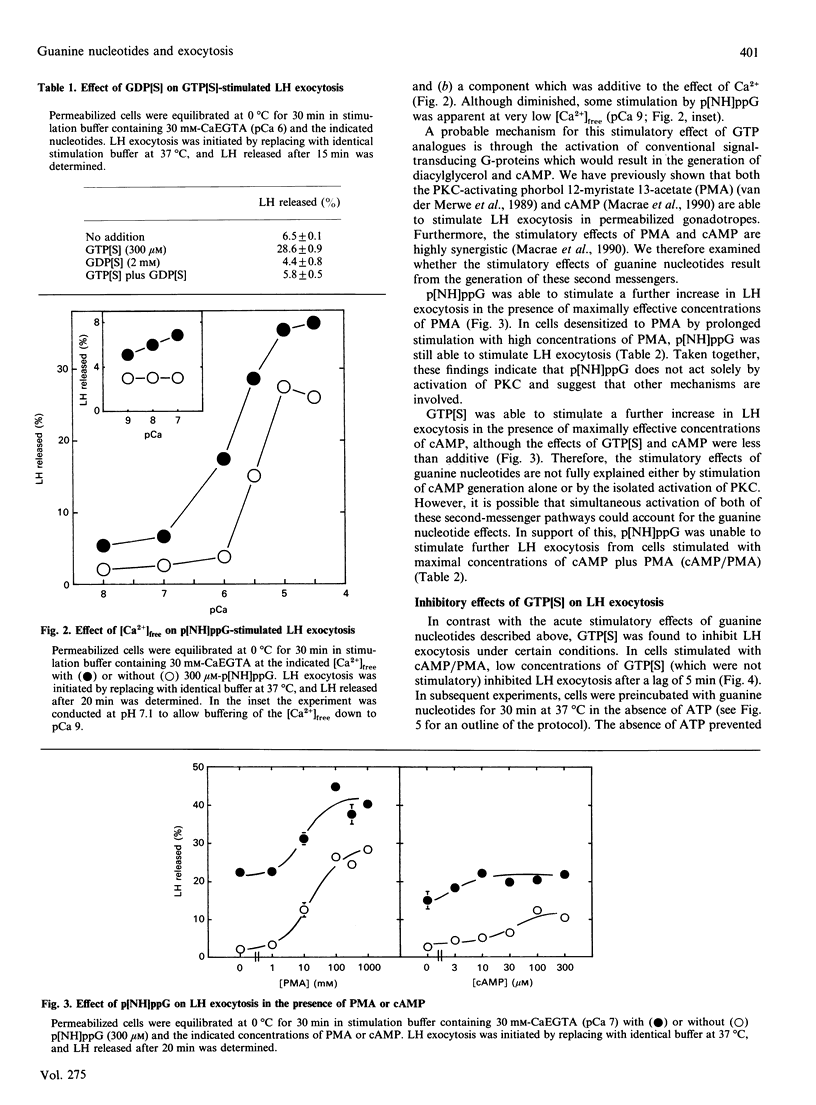
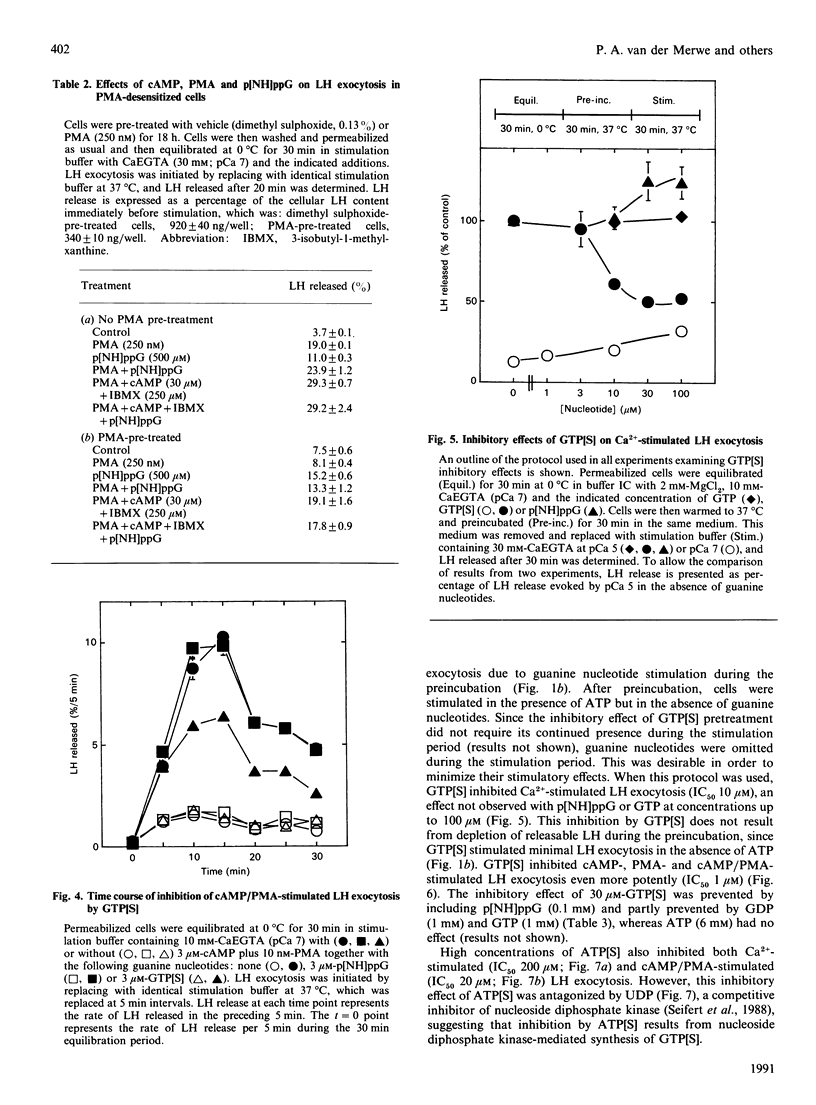
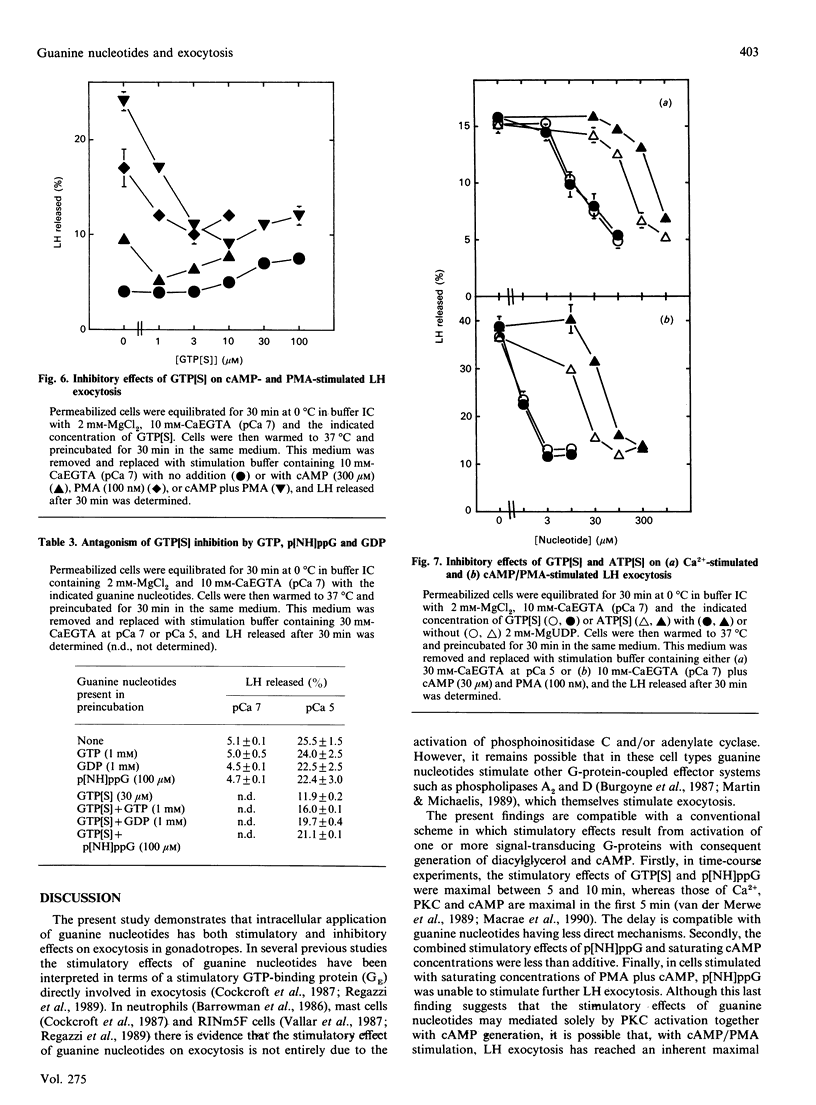
Dual inhibitory and stimulatory actions of guanine nucleotides on luteinizing-hormone (LH) exocytosis were observed in primary sheep gonadotropes permeabilized with staphylococcal alpha-toxin. At resting cytosolic [Ca2+]free (pCa 7), 5'-[gamma-thio]triphosphate (GTP[S]) and guanosine 5'-[beta gamma-imido]triphosphate (p[NH]ppG) stimulated rapid LH exocytosis, which was maximal between 5 and 10 min. GTP[S] and p[NH]ppG had similar potencies (50% of maximum effect at 20-50 microM), but the effect of p[NH]ppG was more prolonged. Experiments carried out in the presence of saturating concentrations of phorbol 12-myristate 13-acetate (PMA), or in PMA-desensitized cells, suggested that stimulation by p[NH]ppG is mediated by a mechanism additional to protein kinase C (PKC) activation. Furthermore, p[NH]ppG stimulated LH exocytosis in the presence of saturating cyclic AMP (cAMP) concentrations, although its effect was less than additive. However, when both PMA and cAMP were present, p[NH]ppG did not stimulate a further increase in the rate of LH exocytosis. In contrast, pretreatment of cells with GTP[S] at low [Ca2+]free markedly inhibited subsequent responses to Ca2+, cAMP, PMA, and cAMP plus PMA. This inhibitory effect required lower GTP[S] concentrations than the stimulatory effect (50% inhibition at 1-10 microM), and was not observed with p[NH]ppG. A similar inhibition was observed with adenosine 5'-[gamma-thio]triphosphate, probably by its conversion into GTP[S]. These results suggest that the stimulatory actions of guanine nucleotides can be accounted for by the combined activation of PKC and generation of cAMP, resulting from activation of conventional signal-transducing GTP-binding proteins. The inhibitory effect of GTP[S] can be clearly distinguished and indicates the involvement of a distinct GTP-binding protein in exocytosis at a site distal to second-messenger generation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnert-Hilger G., Bräutigam M., Gratzl M. Ca2+-stimulated catecholamine release from alpha-toxin-permeabilized PC12 cells: biochemical evidence for exocytosis and its modulation by protein kinase C and G proteins. Biochemistry. 1987 Dec 1;26(24):7842–7848. doi: 10.1021/bi00398a046. [DOI] [PubMed] [Google Scholar]
- Allende J. E. GTP-mediated macromolecular interactions: the common features of different systems. FASEB J. 1988 May;2(8):2356–2367. doi: 10.1096/fasebj.2.8.2452111. [DOI] [PubMed] [Google Scholar]
- Amir-Zaltsman Y., Ezra E., Walker N., Lindner H. R., Salomon Y. Labeling of specific proteins in rat ovarian plasma membranes with [gamma-32 P]GTP. FEBS Lett. 1980 Dec 29;122(2):166–170. doi: 10.1016/0014-5793(80)80429-7. [DOI] [PubMed] [Google Scholar]
- Amir-Zaltsman Y., Salomon Y. Phosphorylation of proteins in rat ovarian plasma membranes by [gamma-32P]GTP: evidence for the formation of a high energy phosphoprotein. Mol Cell Endocrinol. 1989 May;63(1-2):175–187. doi: 10.1016/0303-7207(89)90094-4. [DOI] [PubMed] [Google Scholar]
- Andrews W. V., Staley D. D., Huckle W. R., Conn P. M. Stimulation of luteinizing hormone (LH) release and phospholipid breakdown by guanosine triphosphate in permeabilized pituitary gonadotropes: antagonist action suggests association of a G protein and gonadotropin-releasing hormone receptor. Endocrinology. 1986 Dec;119(6):2537–2546. doi: 10.1210/endo-119-6-2537. [DOI] [PubMed] [Google Scholar]
- Balch W. E. Biochemistry of interorganelle transport. A new frontier in enzymology emerges from versatile in vitro model systems. J Biol Chem. 1989 Oct 15;264(29):16965–16968. [PubMed] [Google Scholar]
- Barrowman M. M., Cockcroft S., Gomperts B. D. Two roles for guanine nucleotides in the stimulus-secretion sequence of neutrophils. Nature. 1986 Feb 6;319(6053):504–507. doi: 10.1038/319504a0. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Damage to cell membranes by pore-forming bacterial cytolysins. Prog Allergy. 1988;40:1–43. [PubMed] [Google Scholar]
- Bittner M. A., Holz R. W., Neubig R. R. Guanine nucleotide effects on catecholamine secretion from digitonin-permeabilized adrenal chromaffin cells. J Biol Chem. 1986 Aug 5;261(22):10182–10188. [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Hewlett E. L., Gilman A. G. Identification of the predominant substrate for ADP-ribosylation by islet activating protein. J Biol Chem. 1983 Feb 25;258(4):2072–2075. [PubMed] [Google Scholar]
- Bourne H. R. Do GTPases direct membrane traffic in secretion? Cell. 1988 Jun 3;53(5):669–671. doi: 10.1016/0092-8674(88)90081-5. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A. Low molecular mass GTP-binding proteins of adrenal chromaffin cells are present on the secretory granule. FEBS Lett. 1989 Mar 13;245(1-2):122–126. doi: 10.1016/0014-5793(89)80204-2. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D. Small GTP-binding proteins. Trends Biochem Sci. 1989 Oct;14(10):394–396. doi: 10.1016/0968-0004(89)90281-8. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Howell T. W., Gomperts B. D. Two G-proteins act in series to control stimulus-secretion coupling in mast cells: use of neomycin to distinguish between G-proteins controlling polyphosphoinositide phosphodiesterase and exocytosis. J Cell Biol. 1987 Dec;105(6 Pt 1):2745–2750. doi: 10.1083/jcb.105.6.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Wallace M. A., Wojcikiewicz R. J. Evidence for involvement of guanine nucleotide-binding regulatory proteins in the activation of phospholipases by hormones. FASEB J. 1988 Jul;2(10):2569–2574. doi: 10.1096/fasebj.2.10.2838362. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D. Involvement of guanine nucleotide-binding protein in the gating of Ca2+ by receptors. Nature. 1983 Nov 3;306(5938):64–66. doi: 10.1038/306064a0. [DOI] [PubMed] [Google Scholar]
- Goud B., Salminen A., Walworth N. C., Novick P. J. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988 Jun 3;53(5):753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- Haslam R. J., Davidson M. M. Guanine nucleotides decrease the free [Ca2+] required for secretion of serotonin from permeabilized blood platelets. Evidence of a role for a GTP-binding protein in platelet activation. FEBS Lett. 1984 Aug 20;174(1):90–95. doi: 10.1016/0014-5793(84)81084-4. [DOI] [PubMed] [Google Scholar]
- Howell T. W., Cockcroft S., Gomperts B. D. Essential synergy between Ca2+ and guanine nucleotides in exocytotic secretion from permeabilized rat mast cells. J Cell Biol. 1987 Jul;105(1):191–197. doi: 10.1083/jcb.105.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Guanine nucleotides and Ca-dependent exocytosis. Studies on two adrenal cell preparations. FEBS Lett. 1985 Sep 23;189(2):345–349. doi: 10.1016/0014-5793(85)81053-x. [DOI] [PubMed] [Google Scholar]
- Li H. C., Simonelli P. F., Huan L. J. Preparation of protein phosphatase-resistant substrates using adenosine 5'-O-(gamma-thio)triphosphate. Methods Enzymol. 1988;159:346–356. doi: 10.1016/0076-6879(88)59035-3. [DOI] [PubMed] [Google Scholar]
- Luini A., De Matteis M. A. Evidence that receptor-linked G protein inhibits exocytosis by a post-second-messenger mechanism in AtT-20 cells. J Neurochem. 1990 Jan;54(1):30–38. doi: 10.1111/j.1471-4159.1990.tb13279.x. [DOI] [PubMed] [Google Scholar]
- Macrae M. B., Davidson J. S., Millar R. P., van der Merwe P. A. Cyclic AMP stimulates luteinizing-hormone (lutropin) exocytosis in permeabilized sheep anterior-pituitary cells. Synergism with protein kinase C and calcium. Biochem J. 1990 Nov 1;271(3):635–639. doi: 10.1042/bj2710635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. W., Michaelis K. P2-purinergic agonists stimulate phosphodiesteratic cleavage of phosphatidylcholine in endothelial cells. Evidence for activation of phospholipase D. J Biol Chem. 1989 May 25;264(15):8847–8856. [PubMed] [Google Scholar]
- Melançon P., Glick B. S., Malhotra V., Weidman P. J., Serafini T., Gleason M. L., Orci L., Rothman J. E. Involvement of GTP-binding "G" proteins in transport through the Golgi stack. Cell. 1987 Dec 24;51(6):1053–1062. doi: 10.1016/0092-8674(87)90591-5. [DOI] [PubMed] [Google Scholar]
- Momayezi M., Lumpert C. J., Kersken H., Gras U., Plattner H., Krinks M. H., Klee C. B. Exocytosis induction in Paramecium tetraurelia cells by exogenous phosphoprotein phosphatase in vivo and in vitro: possible involvement of calcineurin in exocytotic membrane fusion. J Cell Biol. 1987 Jul;105(1):181–189. doi: 10.1083/jcb.105.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oetting M., LeBoff M., Swiston L., Preston J., Brown E. Guanine nucleotides are potent secretagogues in permeabilized parathyroid cells. FEBS Lett. 1986 Nov 10;208(1):99–104. doi: 10.1016/0014-5793(86)81540-x. [DOI] [PubMed] [Google Scholar]
- Regazzi R., Li G., Ullrich S., Jaggi C., Wollheim C. B. Different requirements for protein kinase C activation and Ca2+-independent insulin secretion in response to guanine nucleotides. Endogenously generated diacylglycerol requires elevated Ca2+ for kinase C insertion into membranes. J Biol Chem. 1989 Jun 15;264(17):9939–9944. [PubMed] [Google Scholar]
- Rosenthal W., Hescheler J., Trautwein W., Schultz G. Control of voltage-dependent Ca2+ channels by G protein-coupled receptors. FASEB J. 1988 Sep;2(12):2784–2790. doi: 10.1096/fasebj.2.12.2457531. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. Signal-peptide recognition. GTP and methionine bristles. Nature. 1989 Aug 10;340(6233):433–434. doi: 10.1038/340433a0. [DOI] [PubMed] [Google Scholar]
- Salminen A., Novick P. J. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987 May 22;49(4):527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Seifert R., Rosenthal W., Schultz G., Wieland T., Gierschick P., Jakobs K. H. The role of nucleoside-diphosphate kinase reactions in G protein activation of NADPH oxidase by guanine and adenine nucleotides. Eur J Biochem. 1988 Jul 15;175(1):51–55. doi: 10.1111/j.1432-1033.1988.tb14165.x. [DOI] [PubMed] [Google Scholar]
- Sikdar S. K., Zorec R., Brown D., Mason W. T. Dual effects of G-protein activation on Ca-dependent exocytosis in bovine lactotrophs. FEBS Lett. 1989 Aug 14;253(1-2):88–92. doi: 10.1016/0014-5793(89)80936-6. [DOI] [PubMed] [Google Scholar]
- Ullrich S., Wollheim C. B. GTP-dependent inhibition of insulin secretion by epinephrine in permeabilized RINm5F cells. Lack of correlation between insulin secretion and cyclic AMP levels. J Biol Chem. 1988 Jun 25;263(18):8615–8620. [PubMed] [Google Scholar]
- Vallar L., Biden T. J., Wollheim C. B. Guanine nucleotides induce Ca2+-independent insulin secretion from permeabilized RINm5F cells. J Biol Chem. 1987 Apr 15;262(11):5049–5056. [PubMed] [Google Scholar]
- van der Merwe P. A., Millar R. P., Davidson J. S. Calcium stimulates luteinizing-hormone (lutropin) exocytosis by a mechanism independent of protein kinase C. Biochem J. 1990 Jun 1;268(2):493–498. doi: 10.1042/bj2680493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Merwe P. A., Millar R. P., Wakefield I. K., Davidson J. S. Mechanisms of luteinizing-hormone exocytosis in Staphylococcus aureus-alpha-toxin-permeabilized sheep gonadotropes. Biochem J. 1989 Dec 15;264(3):901–908. doi: 10.1042/bj2640901. [DOI] [PMC free article] [PubMed] [Google Scholar]


