Abstract
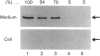
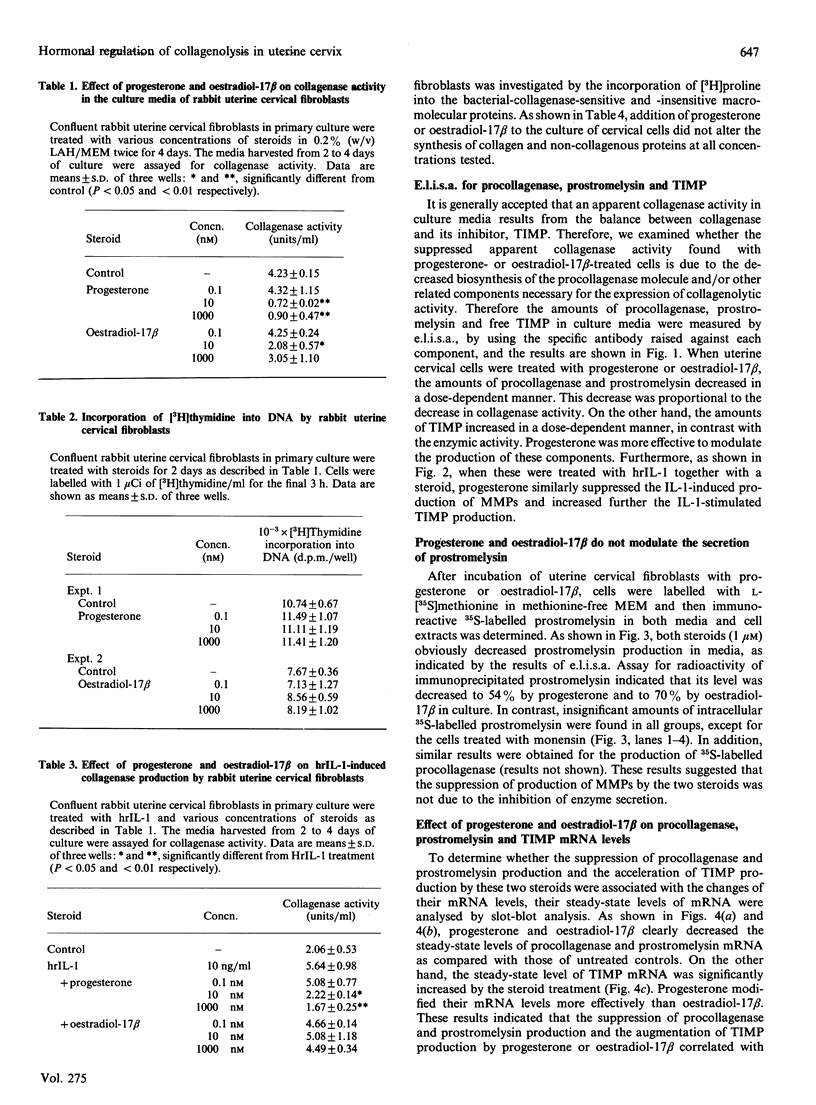
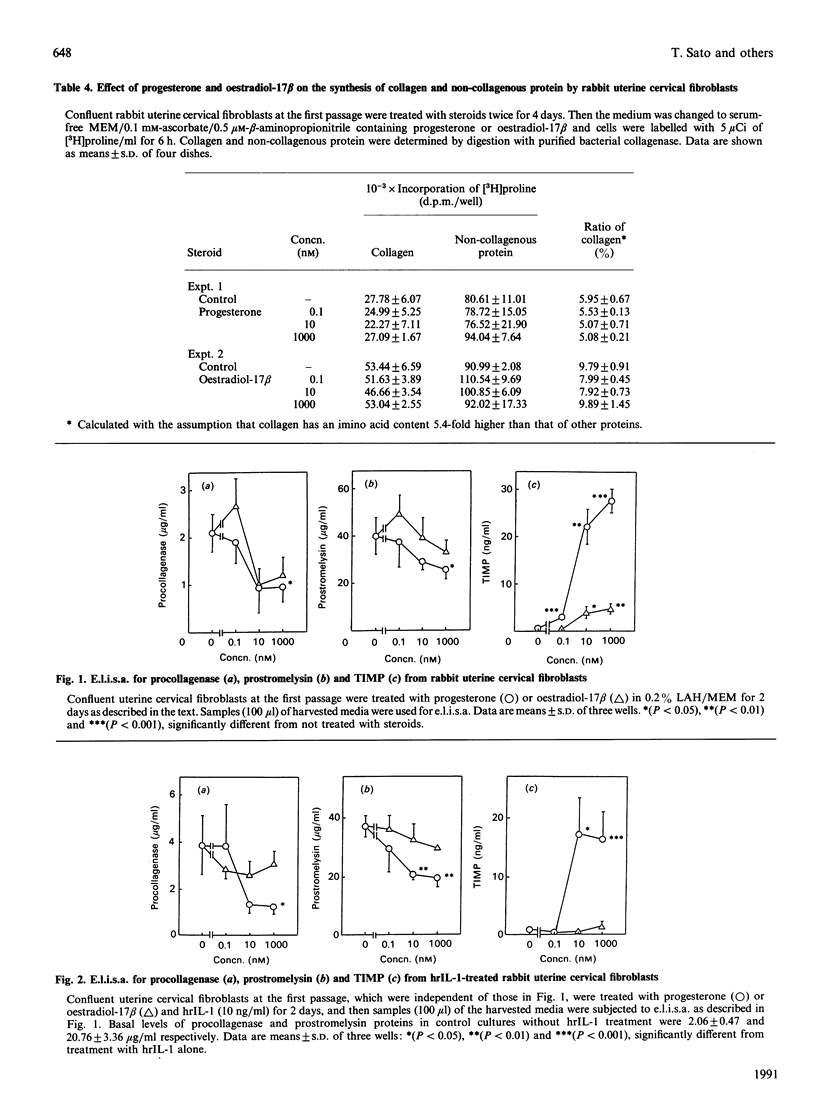
Rabbit uterine cervical fibroblasts produced a large amount of matrix metalloproteinases (MMPs) such as collagenase (MMP-1) and stromelysin (MMP-3) and a small relatively amount of tissue inhibitor of metalloproteinases (TIMP). When cells were treated with progesterone or oestradiol-17 beta, both steroids concurrently decreased the level of procollagenase and prostromelysin in the culture media and the steady-state levels of the respective mRNAs. On the other hand, the level of TIMP in the culture media and the steady-state level of its mRNA were simultaneously increased by these steroids. Similarly, the suppression of production of MMPs and the augmentation of TIMP production by both steroids were observed with interleukin 1 (IL-1)-treated cells, but the action of progesterone was more effective than that of oestradiol-17 beta in the IL-1-untreated and -treated cells. These results suggest that collagenolysis in uterine cervical fibroblasts is negatively regulated by steroid hormones via the acceleration of TIMP production and the suppression of synthesis of MMPs at the pretranslational level.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cartwright E., Murphy G., Sellers A., Reynolds J. J. Collagenase activity from cultured non-gravid rabbit uterus. Biochem Soc Trans. 1977;5(1):229–231. doi: 10.1042/bst0050229. [DOI] [PubMed] [Google Scholar]
- Cruess R. L., Hong K. C. Effect of estrogen on the collagenolytic activity of rat bone. Calcif Tissue Res. 1976 Jun 14;20(3):317–320. doi: 10.1007/BF02546419. [DOI] [PubMed] [Google Scholar]
- DeClerck Y. A. Purification and characterization of a collagenase inhibitor produced by bovine vascular smooth muscle cells. Arch Biochem Biophys. 1988 Aug 15;265(1):28–37. doi: 10.1016/0003-9861(88)90367-0. [DOI] [PubMed] [Google Scholar]
- Grant G. A., Eisen A. Z., Marmer B. L., Roswit W. T., Goldberg G. I. The activation of human skin fibroblast procollagenase. Sequence identification of the major conversion products. J Biol Chem. 1987 Apr 25;262(12):5886–5889. [PubMed] [Google Scholar]
- HE C. S., Wilhelm S. M., Pentland A. P., Marmer B. L., Grant G. A., Eisen A. Z., Goldberg G. I. Tissue cooperation in a proteolytic cascade activating human interstitial collagenase. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2632–2636. doi: 10.1073/pnas.86.8.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. An endopeptidase from rheumatoid synovial tissue culture. Biochim Biophys Acta. 1972 Feb 28;258(2):566–576. doi: 10.1016/0005-2744(72)90249-5. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Welgus H. G., Krane S. M. Regulation of the mammalian collagenases. Coll Relat Res. 1984 Dec;4(6):493–512. doi: 10.1016/s0174-173x(84)80015-1. [DOI] [PubMed] [Google Scholar]
- Ito A., Goshowaki H., Sato T., Mori Y., Yamashita K., Hayakawa T., Nagase H. Human recombinant interleukin-1 alpha-mediated stimulation of procollagenase production and suppression of biosynthesis of tissue inhibitor of metalloproteinases in rabbit uterine cervical fibroblasts. FEBS Lett. 1988 Jul 18;234(2):326–330. doi: 10.1016/0014-5793(88)80109-1. [DOI] [PubMed] [Google Scholar]
- Ito A., Hiro D., Ojima Y., Mori Y. Spontaneous production of interleukin-1-like factors from pregnant rabbit uterine cervix. Am J Obstet Gynecol. 1988 Jul;159(1):261–265. doi: 10.1016/0002-9378(88)90532-7. [DOI] [PubMed] [Google Scholar]
- Ito A., Hiro D., Sakyo K., Mori Y. The role of leukocyte factors on uterine cervical ripening and dilation. Biol Reprod. 1987 Oct;37(3):511–517. doi: 10.1095/biolreprod37.3.511. [DOI] [PubMed] [Google Scholar]
- Ito A., Nagase H. Evidence that human rheumatoid synovial matrix metalloproteinase 3 is an endogenous activator of procollagenase. Arch Biochem Biophys. 1988 Nov 15;267(1):211–216. doi: 10.1016/0003-9861(88)90025-2. [DOI] [PubMed] [Google Scholar]
- Jeffrey J. J., Coffey R. J., Eisen A. Z. Studies on uterine collagenase in tissue culture. II. Effect of steroid hormones on enzyme production. Biochim Biophys Acta. 1971 Oct;252(1):143–149. doi: 10.1016/0304-4165(71)90102-4. [DOI] [PubMed] [Google Scholar]
- Jeffrey J. J. Collagen synthesis and degradation in the uterine deciduoma: regulation of collagenase activity by progesterone. Coll Relat Res. 1981 Apr;1(3):257–268. doi: 10.1016/s0174-173x(81)80003-9. [DOI] [PubMed] [Google Scholar]
- Kitamura K., Ito A., Mori Y., Hirakawa S. Changes in the human uterine cervical collagenase with special reference to cervical ripening. Biochem Med. 1979 Dec;22(3):332–338. doi: 10.1016/0006-2944(79)90020-6. [DOI] [PubMed] [Google Scholar]
- Kodama S., Iwata K., Iwata H., Yamashita K., Hayakawa T. Rapid one-step sandwich enzyme immunoassay for tissue inhibitor of metalloproteinases. An application for rheumatoid arthritis serum and plasma. J Immunol Methods. 1990 Feb 20;127(1):103–108. doi: 10.1016/0022-1759(90)90345-v. [DOI] [PubMed] [Google Scholar]
- Koob T. J., Jeffrey J. J. Hormonal regulation of collagen degradation in the uterus: inhibition of collagenase expression by progesterone and cyclic AMP. Biochim Biophys Acta. 1974 Jun 20;354(1):61–70. doi: 10.1016/0304-4165(74)90053-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Stephens P. E., Smith B. J., Docherty A. J. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J. 1987 Nov 15;248(1):265–268. doi: 10.1042/bj2480265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Reynolds J. J., Werb Z. Biosynthesis of tissue inhibitor of metalloproteinases by human fibroblasts in culture. Stimulation by 12-O-tetradecanoylphorbol 13-acetate and interleukin 1 in parallel with collagenase. J Biol Chem. 1985 Mar 10;260(5):3079–3083. [PubMed] [Google Scholar]
- Murphy G., Werb Z. Tissue inhibitor of metalloproteinases. Identification of precursor forms synthesized by human fibroblasts in culture. Biochim Biophys Acta. 1985 Apr 17;839(2):214–218. doi: 10.1016/0304-4165(85)90039-x. [DOI] [PubMed] [Google Scholar]
- Nagase H., Cawston T. E., De Silva M., Barrett A. J. Identification of plasma kallikrein as an activator of latent collagenase in rheumatoid synovial fluid. Biochim Biophys Acta. 1982 Mar 18;702(1):133–142. doi: 10.1016/0167-4838(82)90036-x. [DOI] [PubMed] [Google Scholar]
- Nagase H., Jackson R. C., Brinckerhoff C. E., Vater C. A., Harris E. D., Jr A precursor form of latent collagenase produced in a cell-free system with mRNA from rabbit synovial cells. J Biol Chem. 1981 Dec 10;256(23):11951–11954. [PubMed] [Google Scholar]
- Okada Y., Watanabe S., Nakanishi I., Kishi J., Hayakawa T., Watorek W., Travis J., Nagase H. Inactivation of tissue inhibitor of metalloproteinases by neutrophil elastase and other serine proteinases. FEBS Lett. 1988 Feb 29;229(1):157–160. doi: 10.1016/0014-5793(88)80817-2. [DOI] [PubMed] [Google Scholar]
- Roswit W. T., Rifas L., Gast M. J., Welgus H. G., Jeffrey J. J. Purification and characterization of human myometrial smooth muscle collagenase. Arch Biochem Biophys. 1988 Apr;262(1):67–75. doi: 10.1016/0003-9861(88)90169-5. [DOI] [PubMed] [Google Scholar]
- Sakyo K., Ito A., Mori Y. Dehydroepiandrosterone sulfate stimulates collagenase synthesis without affecting the rates of collagen and noncollagen protein syntheses by rabbit uterine cervical fibroblasts. Biol Reprod. 1987 Mar;36(2):277–281. doi: 10.1095/biolreprod36.2.277. [DOI] [PubMed] [Google Scholar]
- Sakyo K., Ito A., Mori Y. Effects of dehydroepiandrosterone sulphate on the production of collagenase and gelatinolytic metalloproteinase by rabbit uterine cervical cells in primary cultures. J Pharmacobiodyn. 1986 Mar;9(3):276–286. doi: 10.1248/bpb1978.9.276. [DOI] [PubMed] [Google Scholar]
- Sakyo K., Ito A., Ogawa C., Mori Y. Hormonal control of collagenase inhibitor production in rabbit uterine cervical fibroblast-like cells. Biochim Biophys Acta. 1986 Oct 1;883(3):517–522. doi: 10.1016/0304-4165(86)90292-8. [DOI] [PubMed] [Google Scholar]
- Saus J., Quinones S., Otani Y., Nagase H., Harris E. D., Jr, Kurkinen M. The complete primary structure of human matrix metalloproteinase-3. Identity with stromelysin. J Biol Chem. 1988 May 15;263(14):6742–6745. [PubMed] [Google Scholar]
- Tyree B., Halme J., Jeffrey J. J. Latent and active forms of collagenase in rat uterine explant cultures: regulation of conversion by progestational steroids. Arch Biochem Biophys. 1980 Jun;202(1):314–317. doi: 10.1016/0003-9861(80)90432-4. [DOI] [PubMed] [Google Scholar]
- Valle K. J., Bauer E. A. Biosynthesis of collagenase by human skin fibroblasts in monolayer culture. J Biol Chem. 1979 Oct 25;254(20):10115–10122. [PubMed] [Google Scholar]
- Vater C. A., Nagase H., Harris E. D., Jr Purification of an endogenous activator of procollagenase from rabbit synovial fibroblast culture medium. J Biol Chem. 1983 Aug 10;258(15):9374–9382. [PubMed] [Google Scholar]
- Welgus H. G., Jeffrey J. J., Eisen A. Z. The collagen substrate specificity of human skin fibroblast collagenase. J Biol Chem. 1981 Sep 25;256(18):9511–9515. [PubMed] [Google Scholar]
- Woessner J. F., Jr Total, latent and active collagenase during the course of post-partum involution of the rat uterus. Effect of oestradiol. Biochem J. 1979 Apr 15;180(1):95–102. doi: 10.1042/bj1800095. [DOI] [PMC free article] [PubMed] [Google Scholar]