Abstract
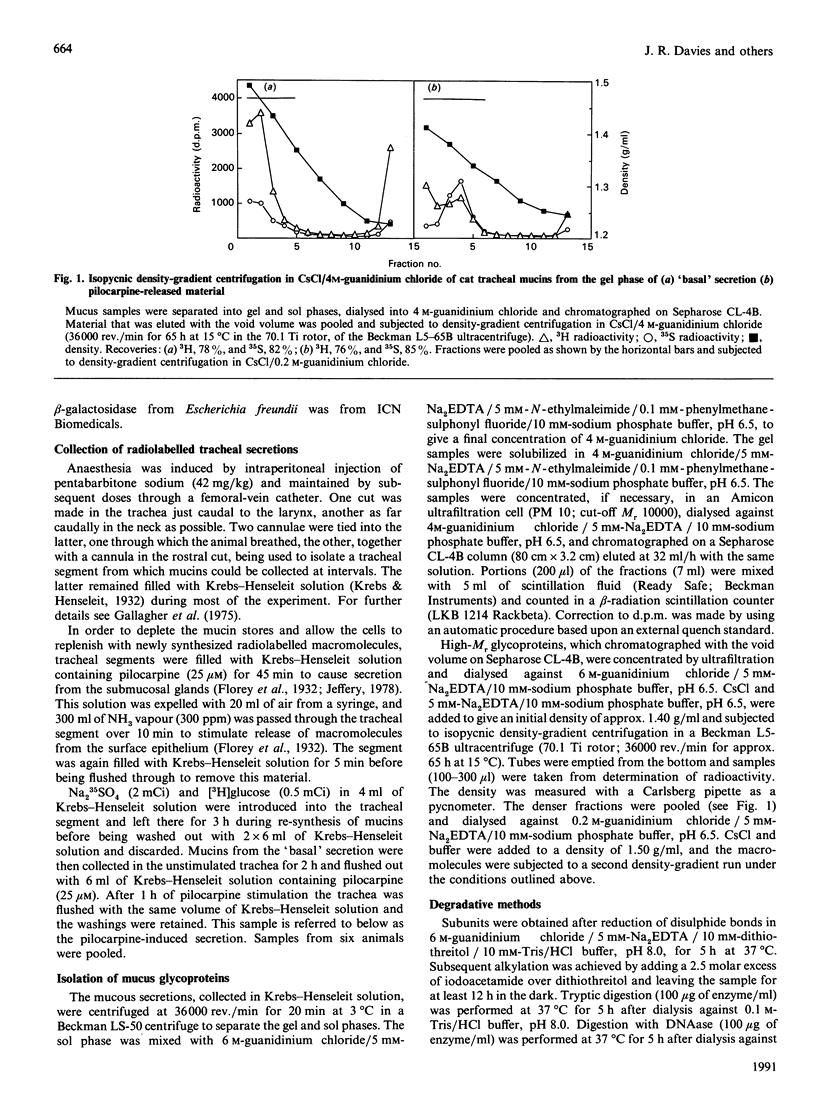
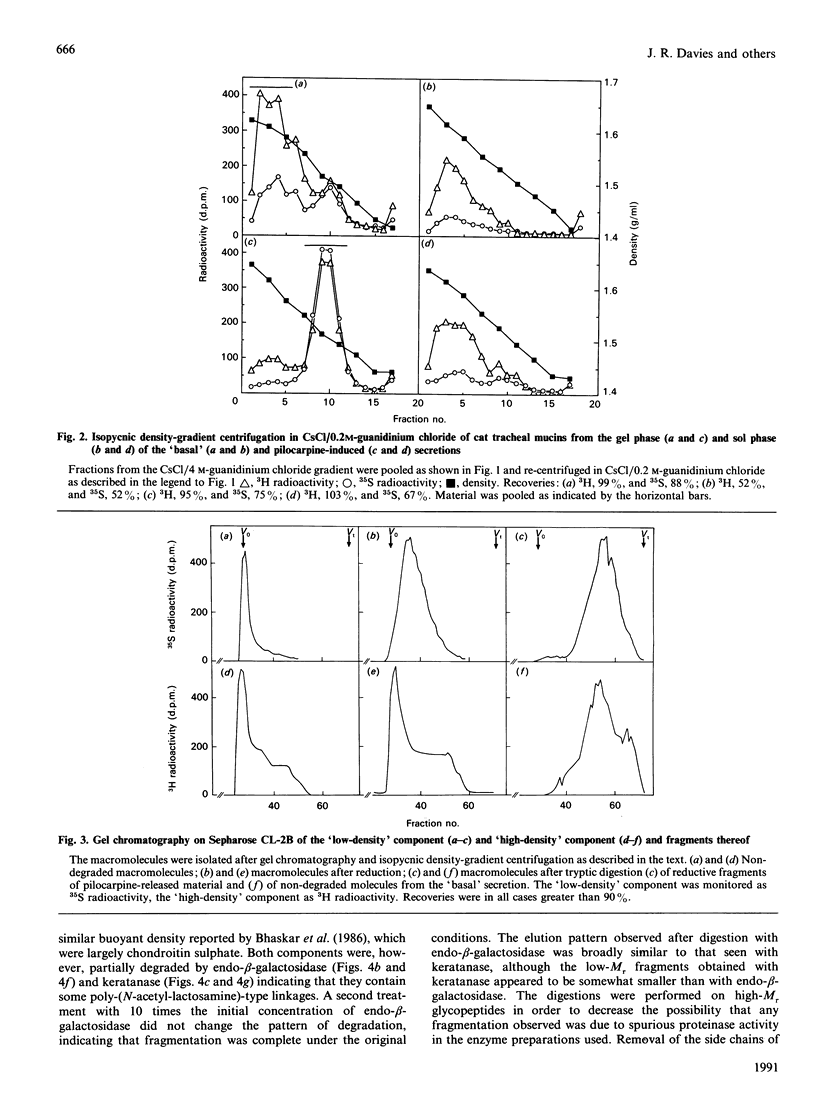
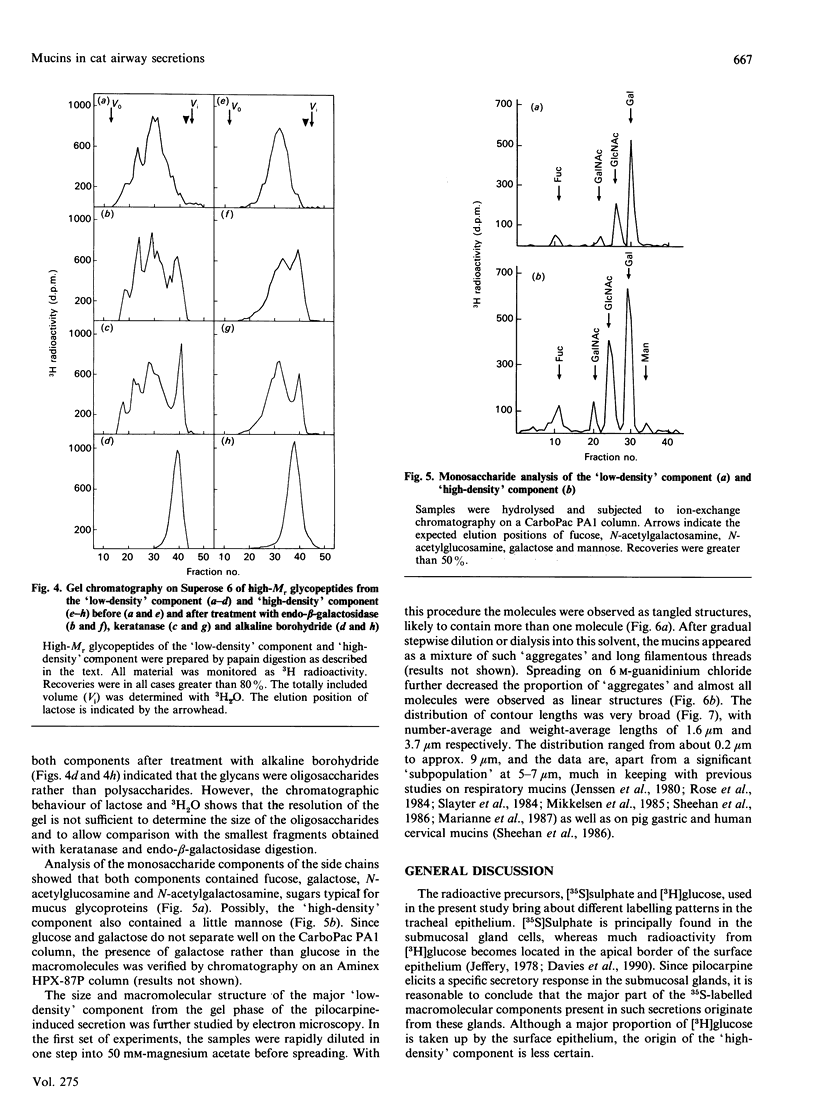
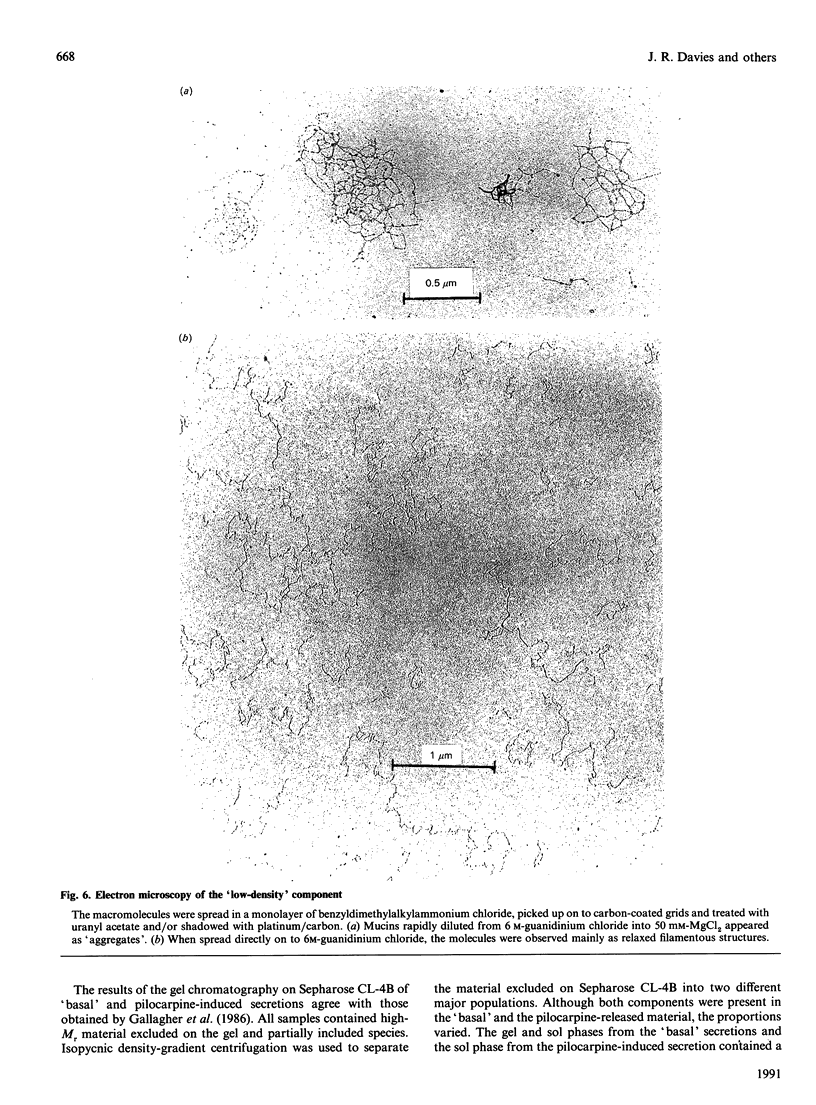
Mucous secretions were obtained from cat tracheas that had received [3H]glucose and [35S]sulphate to radiolabel mucus glycoproteins biosynthetically. Samples were collected under resting ('basal') conditions as well as after pilocarpine stimulation and were separated into gel and sol phases by centrifugation. Macromolecules were partially purified by using gel chromatography on Sepharose CL-4B, and the species that were eluted with the void volume were then separated into two major populations with isopycnic density-gradient centrifugation in CsCl. The major component from the gel phase of pilocarpine-induced secretions had a buoyant density typical of mucins and was observed as linear and apparently flexible chains by electron microscopy. Reduction of disulphide bonds gave subunits that could be further cleaved by trypsin digestion into components of approximately the same size as the high-Mr glycopeptides obtained from other mucins after this treatment. In contrast, the dominant species in the gel phase of the 'basal' secretion had a significantly higher buoyant density than expected for mucins and was largely unaffected by reduction, as studied by gel chromatography. The macromolecules were fragmented by trypsin, suggesting that they contain a polypeptide backbone. This more dense component also predominated in the sol phase both from the 'basal' secretions and from the pilocarpine-released secretions. Digestion with DNAase, chondroitin ABC lyase or heparan sulphate lyase had no effect, which shows that this component is not DNA, a dermatan sulphate/chondroitin sulphate or a heparan sulphate proteoglycan. In contrast, endo-beta-galactosidase and keratanase caused some fragmentation, suggesting that the molecules contain some linkages of the poly-(N-acetyl-lactosamine) type, although the degradation was not as extensive as expected for keratan sulphate. Treatment with alkaline borohydride resulted in extensive fragmentation of the high-Mr glycopeptides from both components, indicating that the glycans were oligosaccharides that were probably O-linked. The monosaccharide compositions of both components were consistent with that expected for mucins. The data are in keeping with the major component from the pilocarpine-stimulated gel secretions being a mucus glycoprotein and the more dense component being a mucin-like molecule, possibly related to the keratanase-sensitive material isolated from canine trachea by Varsano, Basbaum, Forsberg, Borson, Caughey & Nadel [(1987) Exp. Lung Res. 13, 157-184].
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhaskar K. R., O'Sullivan D. D., Opaskar-Hincman H., Reid L. M., Coles S. J. Density gradient analysis of secretions produced in vitro by human and canine airway mucosa: identification of lipids and proteoglycans in such secretions. Exp Lung Res. 1986;10(4):401–422. doi: 10.3109/01902148609058290. [DOI] [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- Carlstedt I., Lindgren H., Sheehan J. K. The macromolecular structure of human cervical-mucus glycoproteins. Studies on fragments obtained after reduction of disulphide bridges and after subsequent trypsin digestion. Biochem J. 1983 Aug 1;213(2):427–435. doi: 10.1042/bj2130427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstedt I., Lindgren H., Sheehan J. K., Ulmsten U., Wingerup L. Isolation and characterization of human cervical-mucus glycoproteins. Biochem J. 1983 Apr 1;211(1):13–22. doi: 10.1042/bj2110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstedt I., Sheehan J. K. Is the macromolecular architecture of cervical, respiratory and gastric mucins the same? Biochem Soc Trans. 1984 Aug;12(4):615–617. doi: 10.1042/bst0120615. [DOI] [PubMed] [Google Scholar]
- Davies J. R., Corbishley C. M., Richardson P. S. The uptake of radiolabelled precursors of mucus glycoconjugates by secretory tissues in the feline trachea. J Physiol. 1990 Jan;420:19–30. doi: 10.1113/jphysiol.1990.sp017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. T., Hall R. L., Phipps R. J., Jeffery P. K., Kent P. W., Richardson P. S. Mucus-glycoproteins (mucins) of the cat trachea: characterisation and control of secretion. Biochim Biophys Acta. 1986 Apr 29;886(2):243–254. doi: 10.1016/0167-4889(86)90142-4. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T., Kent P. W., Passatore M., Phipps R. J., Richardson P. S. The composition of tracheal mucus and the nervous control of its secretion in the cat. Proc R Soc Lond B Biol Sci. 1975 Dec 31;192(1106):49–76. doi: 10.1098/rspb.1975.0151. [DOI] [PubMed] [Google Scholar]
- Jeffery P. K. Structure and function of mucus-secreting cells of cat and goose airway epithelium. Ciba Found Symp. 1978;(54):5–23. doi: 10.1002/9780470720356.ch2. [DOI] [PubMed] [Google Scholar]
- Jenssen A. O., Harbitz O., Smidsrød O. Electron microscopy of mucin from sputum in chronic obstructive bronchitis. Eur J Respir Dis. 1980 Apr;61(2):71–76. [PubMed] [Google Scholar]
- Jones R. The glycoproteins of secretory cells in airway epithelium. Ciba Found Symp. 1978;(54):175–193. doi: 10.1002/9780470720356.ch9. [DOI] [PubMed] [Google Scholar]
- Marianne T., Perini J. M., Lafitte J. J., Houdret N., Pruvot F. R., Lamblin G., Slayter H. S., Roussel P. Peptides of human bronchial mucus glycoproteins. Size determination by electron microscopy and by biosynthetic experiments. Biochem J. 1987 Nov 15;248(1):189–195. doi: 10.1042/bj2480189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen A., Stokke B. T., Christensen B. E., Elgsaeter A. Flexibility and length of human bronchial mucin studied using low-shear viscometry, birefringence relaxation analysis, and electron microscopy. Biopolymers. 1985 Sep;24(9):1683–1704. doi: 10.1002/bip.360240904. [DOI] [PubMed] [Google Scholar]
- Reid L., Bhaskar K., Coles S. Clinical aspects of respiratory mucus. Adv Exp Med Biol. 1982;144:369–391. doi: 10.1007/978-1-4615-9254-9_59. [DOI] [PubMed] [Google Scholar]
- Rose M. C., Voter W. A., Brown C. F., Kaufman B. Structural features of human tracheobronchial mucus glycoprotein. Biochem J. 1984 Sep 1;222(2):371–377. doi: 10.1042/bj2220371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan J. K., Oates K., Carlstedt I. Electron microscopy of cervical, gastric and bronchial mucus glycoproteins. Biochem J. 1986 Oct 1;239(1):147–153. doi: 10.1042/bj2390147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayter H. S., Lamblin G., Le Treut A., Galabert C., Houdret N., Degand P., Roussel P. Complex structure of human bronchial mucus glycoprotein. Eur J Biochem. 1984 Jul 16;142(2):209–218. doi: 10.1111/j.1432-1033.1984.tb08273.x. [DOI] [PubMed] [Google Scholar]
- Thornton D. J., Davies J. R., Kraayenbrink M., Richardson P. S., Sheehan J. K., Carlstedt I. Mucus glycoproteins from 'normal' human tracheobronchial secretion. Biochem J. 1990 Jan 1;265(1):179–186. doi: 10.1042/bj2650179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varsano S., Basbaum C. B., Forsberg L. S., Borson D. B., Caughey G., Nadel J. A. Dog tracheal epithelial cells in culture synthesize sulfated macromolecular glycoconjugates and release them from the cell surface upon exposure to extracellular proteinases. Exp Lung Res. 1987;13(2):157–184. doi: 10.3109/01902148709064316. [DOI] [PubMed] [Google Scholar]



