Abstract
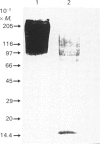
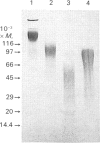
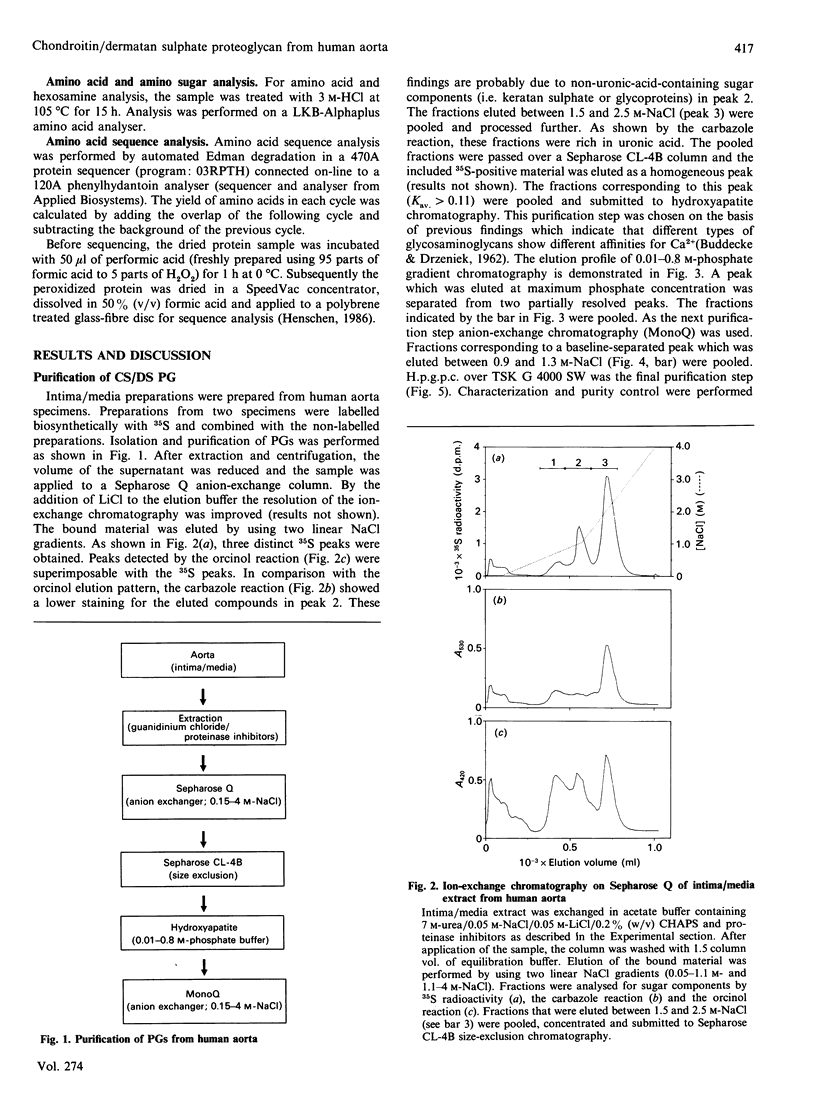
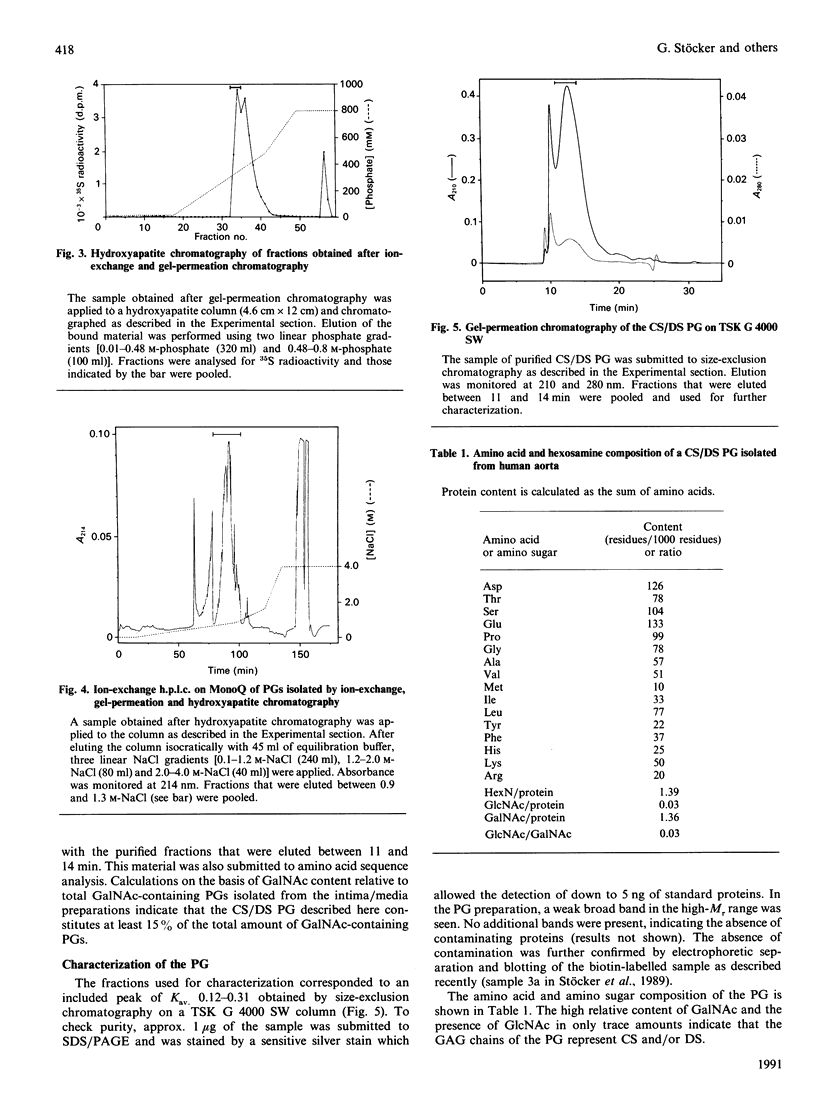
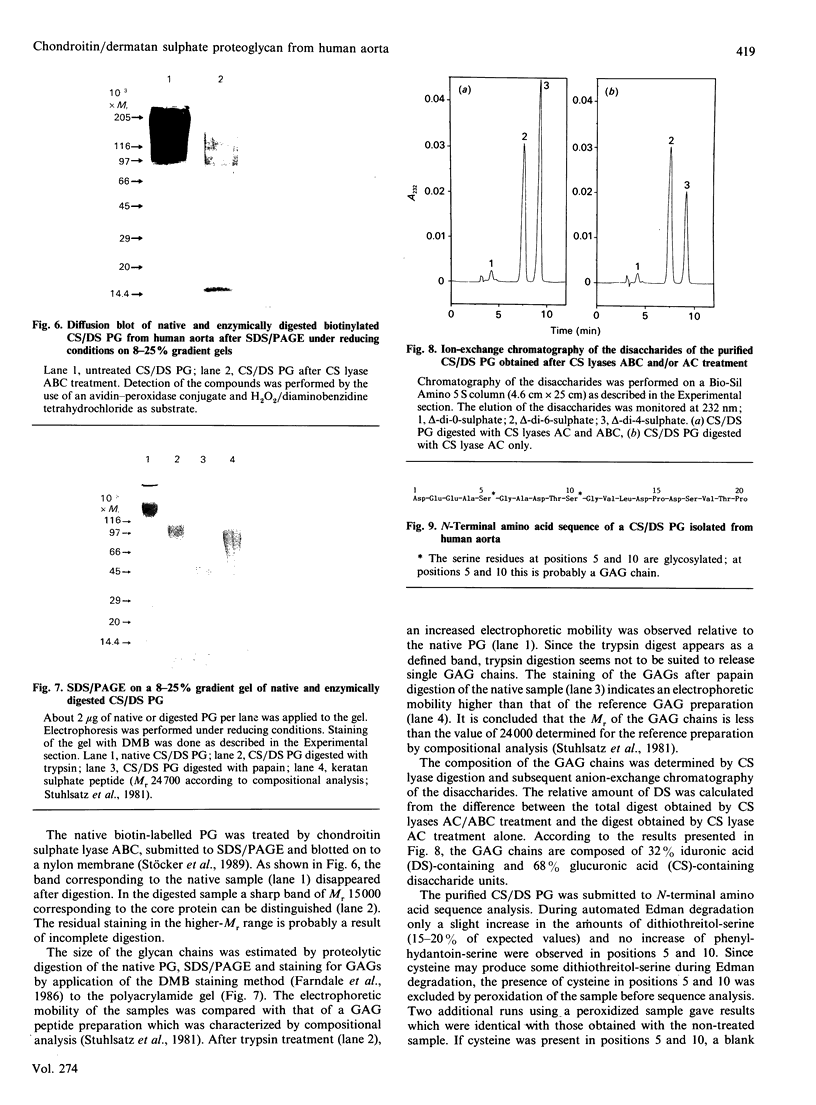
A proteoglycan (PG) was purified to homogeneity from intima/media preparations of human aorta specimens by the following chromatographic steps: Sepharose Q anion exchange, Sepharose CL-4B size exclusion, hydroxyapatite, MonoQ anion exchange and TSK G 4000 SW size exclusion. The purity of the preparation was established by SDS/PAGE using direct staining by silver or Dimethylmethylene Blue, as well as by Western blots of biotin-labelled samples. The electrophoretic mobility of the native PG was less than that of a 200,000-Mr standard protein. After treatment with chondroitin sulphate lyase ABC, a core protein of Mr 15,000 was revealed. The Mr of the glycosaminoglycan (GAG) peptides was less than 24,000, by comparison with a keratan sulphate peptide. The composition of the GAG chains was determined by differential digestion of the PG by chondroitin sulphate lyases AC/ABC or chondroitin sulphate lyase AC alone followed by anion-exchange chromatography of the resulting disaccharides. The GAG chains are composed of approximately one-third of dermatan sulphate and two-thirds chondroitin sulphate disaccharide units. The sequence of the 20 N-terminal amino acids is identical with the sequence previously reported for PG I isolated from human developing bone [Fisher, Termine & Young (1989) J. Biol. Chem. 264, 4571-4576]. The assignment of glycosylation sites to the serine residues in positions 5 and 10 was confirmed. The findings indicate that the chondroitin sulphate/dermatan sulphate PG is a major PG in intima/media preparations of human aorta and represents a biglycan-type PG.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- BUDDECKE E., DRZENIEK R. [Stability constants of the calcium complexes of acid mucopolysaccharides]. Hoppe Seylers Z Physiol Chem. 1962 May 4;327:49–64. doi: 10.1515/bchm2.1962.327.1.49. [DOI] [PubMed] [Google Scholar]
- Farndale R. W., Buttle D. J., Barrett A. J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986 Sep 4;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Fisher L. W., Hawkins G. R., Tuross N., Termine J. D. Purification and partial characterization of small proteoglycans I and II, bone sialoproteins I and II, and osteonectin from the mineral compartment of developing human bone. J Biol Chem. 1987 Jul 15;262(20):9702–9708. [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D., Young M. F. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989 Mar 15;264(8):4571–4576. [PubMed] [Google Scholar]
- Hedbom E., Heinegård D. Interaction of a 59-kDa connective tissue matrix protein with collagen I and collagen II. J Biol Chem. 1989 Apr 25;264(12):6898–6905. [PubMed] [Google Scholar]
- Heinegård D., Björne-Persson A., Cöster L., Franzén A., Gardell S., Malmström A., Paulsson M., Sandfalk R., Vogel K. The core proteins of large and small interstitial proteoglycans from various connective tissues form distinct subgroups. Biochem J. 1985 Aug 15;230(1):181–194. doi: 10.1042/bj2300181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heukeshoven J., Dernick R. Improved silver staining procedure for fast staining in PhastSystem Development Unit. I. Staining of sodium dodecyl sulfate gels. Electrophoresis. 1988 Jan;9(1):28–32. doi: 10.1002/elps.1150090106. [DOI] [PubMed] [Google Scholar]
- Kato M., Oike Y., Suzuki S., Kimata K. Selective removal of heparan sulfate chains from proteoheparan sulfate with a commercial preparation of heparitinase. Anal Biochem. 1985 Aug 1;148(2):479–484. doi: 10.1016/0003-2697(85)90255-6. [DOI] [PubMed] [Google Scholar]
- Lindblom A., Carlstedt I., Fransson L. A. Identification of the core proteins in proteoglycans synthesized by vascular endothelial cells. Biochem J. 1989 Jul 1;261(1):145–153. doi: 10.1042/bj2610145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H. E., Hoffmann-Posorske E., Korte H., Heilmeyer L. M., Jr Sequence analysis of phosphoserine-containing peptides. Modification for picomolar sensitivity. FEBS Lett. 1986 Aug 11;204(1):61–66. doi: 10.1016/0014-5793(86)81388-6. [DOI] [PubMed] [Google Scholar]
- Murata K., Kotake C., Motoyama T. Collagen species in human aorta: with special reference to basement membrane-associated collagens in the intima and media and their alteration with atherosclerosis. Artery. 1987;14(4):229–247. [PubMed] [Google Scholar]
- Murata K., Motoyama T. Collagen species in various sized human arteries and their changes with intimal proliferation. Artery. 1990;17(2):96–106. [PubMed] [Google Scholar]
- Oike Y., Kimata K., Shinomura T., Nakazawa K., Suzuki S. Structural analysis of chick-embryo cartilage proteoglycan by selective degradation with chondroitin lyases (chondroitinases) and endo-beta-D-galactosidase (keratanase). Biochem J. 1980 Oct 1;191(1):193–207. doi: 10.1042/bj1910193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Structure and biology of proteoglycans. Annu Rev Cell Biol. 1988;4:229–255. doi: 10.1146/annurev.cb.04.110188.001305. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Orford C. R. Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem J. 1981 Jul 1;197(1):213–216. doi: 10.1042/bj1970213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988 Jun 1;252(2):313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlsatz H. W., Hirtzel F., Keller R., Cosma S., Greiling H. Studies on the polydispersity and heterogeneity of proteokeratan sulfate from calf and porcine cornea. Hoppe Seylers Z Physiol Chem. 1981 Jul;362(7):841–852. doi: 10.1515/bchm2.1981.362.2.841. [DOI] [PubMed] [Google Scholar]
- Stöcker G., Lückge J., Greiling H., Wagener C. Characterization of biotin-labeled proteoglycans by electrophoretic separation on minigels and blotting onto nylon membranes prior and after enzymatic digestion. Anal Biochem. 1989 Jun;179(2):245–250. doi: 10.1016/0003-2697(89)90123-1. [DOI] [PubMed] [Google Scholar]
- Wight T. N. Proteoglycans in pathological conditions: atherosclerosis. Fed Proc. 1985 Feb;44(2):381–385. [PubMed] [Google Scholar]
- Ylä-Herttuala S., Sumuvuori H., Karkola K., Möttönen M., Nikkari T. Glycosaminoglycans in normal and atherosclerotic human coronary arteries. Lab Invest. 1986 Apr;54(4):402–407. [PubMed] [Google Scholar]