Abstract
Key Clinical Message
Differentiating Adult‐Onset Still's Disease (AOSD) from lymphoma is challenging. A 23‐year‐old female presented with polyarthralgia, fever, rash, lymphadenopathy, and abnormal labs. She met AOSD criteria and was diagnosed with non‐Hodgkin lymphoma. Treatment led to improvement.
Abstract
The differentiation between Adult‐onset Still's Disease (AOSD) and lymphoma remains challenging in our context. The Faustrel score facilitates the simultaneous diagnosis of these conditions. A 23‐year‐old woman presented with chronic polyarthralgia in a febrile context. A physical examination revealed peripheral joint syndrome, a maculopapular rash on the trunk, and peripheral lymphadenopathy. Laboratory tests showed neutrophils leukocytosis, hyperferritinemia, and a glycosylated ferritin fraction of 12%. A lymph node biopsy revealed a malignant non‐Hodgkin lymphoma, and the patient met the diagnostic criteria for AOSD according to Faustrel. She received corticosteroid therapy and four courses of Rituximab. After 6 months, clinical and biological improvements were noted. It is essential to consider the possibility of an association between atypical clinical presentations of lymphoma and AOSD.
Keywords: adult‐onset Still's disease, ferritin, glycosylated ferritin fraction, lymphoma
1. INTRODUCTION
In 1971, Bywaters described the first cases of AOSD, a clinical entity considered to be the adult equivalent (i.e., in patients over 17 years of age at onset) of systemic juvenile idiopathic arthritis described by Still a century ago. 1 Adult‐onset Still's disease (AOSD) is a rare autoinflammatory condition resulting from a multigenic interaction, with an incidence of 1 to 34 cases per million inhabitants, generally with a good prognosis. 2 AOSD and lymphomas exhibit notable clinical similarities, making it challenging to differentiate between these two conditions. Confirming a diagnosis of AOSD requires the exclusion of infectious, hematologic, autoimmune, and neoplastic causes. 3 However, a more recent scoring system, the Faustrel score, does not require exclusion criteria; it has a sensitivity of 80.6% and a specificity of up to 98.5%, which is superior to the Yamaguchi score. 2 The Faustrel score considers glycosylated ferritin and does not account for exclusion criteria, allowing for the diagnosis of such an association. In medical literature, the association between AOSD and lymphoma is rarely described, with even fewer reports of the simultaneous diagnosis of these conditions. This study describes a concurrent diagnosis of AOSD and lymphoma in a patient.
2. CASE REPORT
2.1. Case history
A 23‐year‐old woman patient, nulligravida, no tobacco or drug history and no personal or familial pathological history, presented with polyarthralgia affecting both large and small joints, occurring intermittently for the past 8 months, accompanied by recurrent fever primarily in the afternoons.
2.2. Examination
Physical examination revealed hemodynamic stability (Blood pressure at 110/78 mmHg, heart rate at 60 beats per minute, respiratory rate at 18 cycles per minute, peripheral oxygen saturation at 98% on room air, pulse at 60 pulses per minute.), a mild fever at 38.3°C, and bilateral polyarthralgia involving the wrists, metacarpophalangeal, and proximal and distal interphalangeal joints. Additionally, a non‐pruritic maculopapular rash on the trunk had appeared 2 weeks prior. A tumoral syndrome was noted, characterized by bilateral, painless, mobile axillary and cervical lymphadenopathy, the largest measuring 5 cm in its greatest dimension, along with stage 2 splenomegaly (Hackett's classification) and moderate hepatomegaly.
2.3. Investigation
Laboratory tests revealed leukocytosis with a white blood cell count of 13.810/μL predominantly neutrophils, hyperferritinemia at 3550 ng/mL, and a glycosylated ferritin fraction of 12%. The biopsy of the accessory salivary glands with histological examination was normal. Further biological evaluations are summarized in Table 1.
TABLE 1.
Summary of biological tests in our case.
| Parameter | Value | Normal Value |
|---|---|---|
| White Blood Cells (/mm3) | 13,810 | 4000–10,000 |
| Neutrophils (/mm3) | 8500 | 2000–7500 |
| Lymphocytes (/mm3) | 1250 | 1000–4000 |
| Hemoglobin level (g/dl) | 11.1 | 12–16 (female) |
| Mean corpuscular volume (fl) | 76 | 80–100 |
| Platelets (/mm3) | 199,000 | 150,000–450,000 |
| Creatinine (μmol/L) | 67 | 44–110 (female) |
| C Reactive Protein (mg/L) | 48 | <6 |
| Aspartate aminotransferase (IU/L) | 26.7 | 10–40 |
| Alanine aminotransferase (IU/L) | 20.36 | 7–56 |
| Lactate dehydrogenase (IU/L) | 206 | 140–280 |
| Creatinine phosphokinase (IU/L) | 242 | 20–200 |
| Glycolysed fraction of ferritin (%) | 12 | <10 |
| Erythrocyte sedimentation rate (mm) | 28 | 0–20 (female) |
| Serum ferritin (ng/ml) | 3350 | 30–400 |
| 24‐hour proteinuria (g/24 h) | 0.211 | <0.15 |
| Rheumatoid factors (IU/l) | < 8 | <14 |
| Anti‐citrullinated protein antibodies (IU/L) | < 3.5 | <20 |
| Angiotensin converting enzyme (IU/L) | 15 | 8–52 |
| anti‐ds‐DNA antibody (UI/mL) | 5 | < 20 |
| Antinuclear antibodies (U) | 0.1 | ≤1.0 |
| Antinuclear Factors (U) | 0.1 | < 1.0 |
| Anti‐neutrophil cytoplasmic antibody (AU/mL) | 6 | ≤19 |
| Anti‐Ro (U) | 0.1 | < 1.0 |
2.4. Diagnosis
Initial diagnosis considered was AOSD based on the Faustrel criteria (Table 2). An axillary lymph node biopsy presented as a cluster of micro‐lymphadenopathies. A total of six lymph nodes were observed, measuring between 0.5 cm and 3 cm in their largest dimension. The nodes were grayish and had a soft consistency (Figure 1). No necrosis or hemorrhage was observed on the surface or upon sectioning of the nodes. The histological immunohistochemical study identified a small B‐cell non‐Hodgkin lymphoma (NHL) grade I (Figure 2A). The immunohistochemical study identified positive staining with anti‐CD20, Bcl2, Bcl1, Bcl6, and CD10 antibodies. The Ki67 proliferation index was evaluated at 40% (Figure 2B). Consequently, a concurrent diagnosis of AOSD associated with small B‐cell NHL was established.
TABLE 2.
Faustrel criteria present in our patient.
| Fautrel criteria | Present criteria |
|---|---|
| Major criteria | |
| Hectic fever ≥39°C | + |
| Arthralgia | + |
| Transient erythema | Absent |
| Pharyngitis | Absent |
| Neutrophil count >80% | + |
| Glycosylated ferritin ≤20% | + |
| Minor criteria | |
| Maculopapular rash | + |
| Leukocyte count >10.000/mm3 | + |
Note: The diagnosis is based on the presence of at least four major criteria or three major criteria combined with two minor criteria.
FIGURE 1.
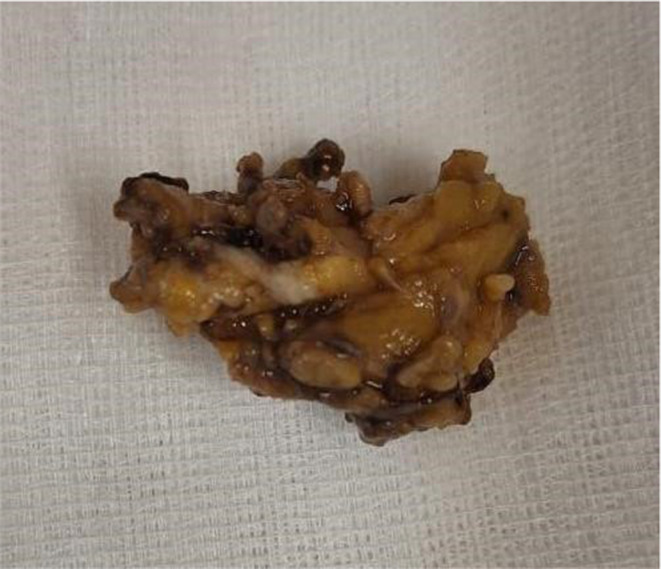
Left axillary adenopathy excision specimen.
FIGURE 2.
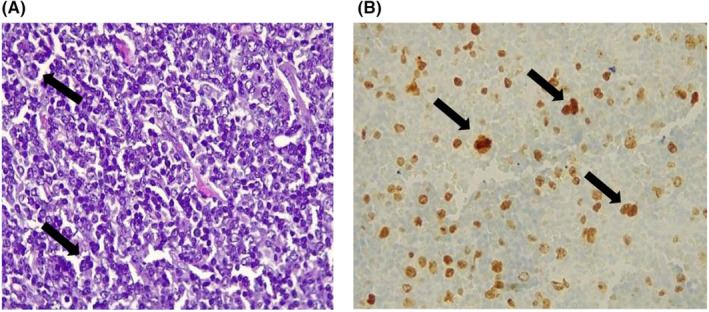
(A) Histological examination of the ganlion shows a lymphoid proliferation of small cells, with cytonuclear atypia such as anisocytosis and anisokaryosis (G400). (B) Immunohistochemical image (Ki67 immunostaining, G400) showing intense nuclear labelling of tumor cells with some mitotic figures.
2.5. Treatment
The patient was treated with methylprednisolone at a dose of 32 mg per day, tapered by 10% every 10 days for AOSD and NHL, along with 4 courses of rituximab administered weekly for NHL.
2.6. Outcomes
Clinical and biological improvement was observed, achieving remission within 6 months.
3. DISCUSSION
AOSD pathogenesis lies at the intersection of inflammasomopathies and lymphohistiocytic activation. 4 The diagnosis of AOSD is typically based on the classic triad of symptoms, including fever, polyarthralgia, and a transient rash. Despite this, it remains challenging due to the absence of specific tests and characteristic clinical manifestations, its low incidence, and the diversity of its clinical presentation. This disease primarily affects young adults, predominantly those aged 16 to 35, and is observed in both sexes without geographic distinction. 4
The association between AOSD and neoplasms has been reported in a few cases, including cases of lymphomas. However, the simultaneous occurrence of these two pathologies is rarely described. This review of the literature on the simultaneous diagnosis of AOSD and lymphoma worldwide was conducted using the MEDLINE (via PUBMED) and Google Scholar databases. This research was supplemented by a manual search of the bibliographic references of all potentially eligible full‐text articles. Publications were identified using the following keywords: “AOSD AND lymphoma AND association.” We found two articles referenced in English, both of which were case reports where the diagnosis of AOSD and lymphoma was made simultaneously. Cases diagnosed post‐mortem or during the course of the disease were excluded. Table 3 summarizes the different cases described in the literature as well as our case based on year, authors, age, sex of patients, type of lymphoma, treatments, and outcomes (Table 3).
TABLE 3.
Summary table of the different cases in our literature review.
| N° | Authors/Year | Age/ Sex | Symptoms | Serum ferritin (ng/ml)/Ferritin glycolytic fraction (%) | Type of lymphoma | Treatment by the AOSD | Treatment of lymphoma | Evolution |
|---|---|---|---|---|---|---|---|---|
| 1 | Isimbaldi et al/2000 5 | 18 /M | Recurrent fever, skin rash and arthralgia | 12,500/ on available | LMNH (angiotropic T) | Corticotherapy | Not available | Death due to multiple organ failure |
| 2 | Kato et al/2009 6 | 25/M |
Fever, sore throat, oligoarthralgia and skin lesions |
727.4/ not available | LMNH (nasal NK) | Corticosteroid therapy | Chemotherapy | Not available |
| 3 | Bayala et al/2024 | 23/F |
Recurrent fever and polyarthritis |
3550/12 | LMNH (small B cells) | Corticosteroid therapy | Chemotherapy | Complete remission of lymphoma, AOSD: controlled |
Abbreviations: NHL, non‐Hodgkin's lymphoma; NK, natural killer.
The association of AOSD with other malignant pathologies remains controversial, but since the appearance of the Fautrel criteria in 2002, it is considered possible because these criteria give significant importance to serum ferritin and the glycosylated fraction of ferritin. The mechanisms underlying hyperferritinemia in AOSD are not fully understood. These ferritin levels are generally higher than those observed during inflammation and hepatic cytolysis. 7 Some authors suggest an elevated ferritin level at 4 to 5 times the normal level with high sensitivity. 2 A very high ferritin level lacks specificity, whereas the drastically reduced glycosylated ferritin level is more specific, making it a major criterion for guiding the diagnosis. 2 When this criterion is considered, the diagnosis is retrospectively confirmed, especially in the absence of underlying infectious or neoplastic pathology. 7 This score is useful for supporting the diagnosis in clinical practice, but the major criterion of glycosylated ferritin less than 20% is not pathognomonic for AOSD.
Hyperferritinemia is present in other pathologies such as cancers, chronic inflammatory rheumatism, and infections, but the decrease in the glycosylated fraction of ferritin is highly specific to AOSD. Indeed, several studies have examined the role of serum ferritin in lymphomas. For instance, Yoh et al. revealed that serum ferritin levels were significantly higher in patients with Hodgkin's lymphoma compared to controls. Thus, ferritin level appears to be a useful biomarker for the diagnosis and prognosis of lymphomas. 8 However, in cases of AOSD, the peculiarity of hyperferritinemia is its elevation to five times the normal level, as in our case, where it was elevated to 12 times the normal level. In contrast, in our literature review, only the first patient had such an elevation of the ferritin level, being 41 times the normal level.
Regarding the glycosylated fraction of ferritin in lymphomas, studies have shown that it is elevated in patients with lymphomas and may correlate with the disease stage and treatment response. 9 The exact mechanism by which the glycosylated fraction is elevated in lymphomas is not fully understood. However, it is believed to be related to the increased glucose metabolism in tumor cells. 9 Tumor cells require large amounts of glucose to support their growth and proliferation, and glycolysis is the primary metabolic pathway used to break down glucose. 9 Thus, in our patient, the decrease in the glycosylated fraction of ferritin to 12% was not related to the lymphoma but rather suggested AOSD. It was one of our major criteria for diagnosing AOSD in our patient according to the Faustrel criteria. This measurement was not performed on the other two patients in our literature review because their diagnostic criterion was that of Yamaguchi.
The diagnosis of lymphoma was based on the biopsy and histological and immunohistochemical study of the lymph nodes in our case, as in other cases described in the literature. The lymphoma lesions in our case differed from those typically seen in AOSD, which are generally paracortical, mixed, diffuse, necrotic, or follicular. 10 The follicular pattern is characterized by extensive hyperplasia of lymphoid follicles; the paracortical pattern by proliferation of paracortical zones; the diffuse pattern by diffuse hyperplasia of paracortical zones, without observation of lymphoid follicle structures; the necrotic pattern by proliferative expansion of paracortical zones, focal sparse necrosis, and nuclear fragmentation; and mixed patterns by lymphoid follicles or diffuse areas associated with paracortical zones. 10
Some cases of the association between AOSD and lymphoma are described in the literature, in which AOSD precedes lymphoma or vice versa. Our case report is the first to describe a simultaneous diagnosis of AOSD and small B‐cell type LMNH. It is the third such association reported in the literature. Remarkably, our patient achieved remission from both diseases. Notably, this is the first report of such an association in a black african individual. However, the diagnosis of these cases was made using Yamaguchi's diagnostic criteria, which raises the question whether these were pseudo‐AOSD cases. Nonetheless, patients suffering from autoimmune diseases are more likely to develop lymphoma. 11 Particularly, Sjögren's syndrome appears to significantly increase the risk, with a prevalence of lymphoma up to 15 times higher. 11
For the treatment of AOSD, all three patients received corticosteroid therapy as symptomatic treatment. Certain biologics, such as anakinra and canakinumab, are indicated as maintenance therapy in cases of corticosteroid failure. 12 This highlights the importance of ruling out other differential diagnoses of AOSD, as some AOSD treatments can worsen the course of these differential diagnoses, particularly infectious diseases.
4. CONCLUSION
The coexistence of AOSD and lymphoma poses significant diagnostic and therapeutic challenges. The immunopathological connections between AOSD and lymphoma remain poorly understood, particularly given the extremely rare reported cases. Nevertheless, it is crucial to remain vigilant in the face of any anomalies in the clinical presentation of lymphoma or AOSD.
AUTHOR CONTRIBUTIONS
Yannick Laurent Tchenadoyo Bayala: Conceptualization; methodology; supervision; validation; visualization; writing – original draft. Issa Ouedraogo: Visualization; writing – original draft. Hervé Eric Mourfou: Visualization; writing – original draft. Bakoubassé Aïssata Son: Validation. Wendyam Nadège Yameogo: Validation. AYOUBA TINNI Ismael: Validation. Joelle Wendlassida Stéphanie Zabsonre/ Tiendrebeogo: Supervision. Dieu‐Donné Ouedraogo: Supervision.
FUNDING INFORMATION
No specific funding was received from any bodies in the public, commercial or not‐ for‐ profit sectors to carry out the work described in this article.
CONSENT
I confirm that written patient consent has been signed and collected in accordance with the journal's patient consent policy, and that I have added a patient consent statement asserting this at the bottom of the manuscript's title page. I will retain the original written consent form and provide it to the Publisher if requested.
ACKNOWLEDGMENTS
We acknowledge Prof. Aimé Sosthène Ouedraogo and Dr. Ezeckiel Bocovo for providing histological images.
Bayala YLT, Ouedraogo I, Mourfou HE, et al. Simultaneous diagnosis of adult‐onset Still's disease and lymphoma: A case report and systematic review of the literature. Clin Case Rep. 2024;12:e9509. doi: 10.1002/ccr3.9509
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Smaali J, Khattabi AE, Qatni ME, et al. Maladie de Still de l'adulte et lymphome: une association rare. Pan Afr Med J. 2020;36(1):55‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chahine B, Luthier F. Valeur diagnostique de l'hyperferritinémie et de la ferritine glycosylée dans la maladie de Still de l'adulte: 3 observations. Presse Med. 2005;34(13):928‐932. [DOI] [PubMed] [Google Scholar]
- 3. Lebrun D, Mestrallet S, Dehoux M, et al. Validation of the Fautrel classification criteria for adult‐onset Still's disease. Semin Arthritis Rheum. 2018;47(4):578‐585. [DOI] [PubMed] [Google Scholar]
- 4. Giacomelli R, Ruscitti P, Shoenfeld Y. A comprehensive review on adult onset Still's disease. J Autoimmun. 2018;93(12):24‐36. [DOI] [PubMed] [Google Scholar]
- 5. Isimbaldi G, Corral L, Songia S, Valente MG, de Bianchi S, Biondi A. An unusual presentation of a case of T cell angiotropic (intravascular) lymphoma. Leukemia. 2000;14(12):2321‐2322. [DOI] [PubMed] [Google Scholar]
- 6. Kato T, Tanabe J, Kanemoto M, Kobayashi C, Morita S, Karahashi T. A case of extranodal NK/T‐cell lymphoma, nasal type mimicking typical manifestations of adult‐onset Still's disease (AOSD) with hemophagocytic syndrome: diagnostic consideration between malignant lymphoma without lymphadenopathy and AOSD. Mod Rheumatol. 2009;19(6):675‐680. [DOI] [PubMed] [Google Scholar]
- 7. Fautrel B, Moël GL, Saint‐Marcoux B, et al. Diagnostic value of ferritin and glycosylated ferritin in adult onset Still's disease. J Rheumatol. 2021;28(3):322‐329. [PubMed] [Google Scholar]
- 8. Yoh KA, Lee HS, Park LC, et al. The prognostic significance of elevated levels of serum ferritin before chemotherapy in patients with non‐Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk. 2014;14(1):43‐49. [DOI] [PubMed] [Google Scholar]
- 9. Shen Z, Zhang S, Zhang M, et al. The addition of ferritin enhanced the prognostic value of international prognostic index in diffuse large B‐cell lymphoma. Front Oncol. 2022;11(6):63‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang Z, Xu H, Min Q, et al. Adult‐onset Still's disease with multiple lymphadenopathy: a case report and literature review. Diagn Pathol. 2021;16(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smedby KE, Hjalgrim H, Askling J, et al. Autoimmune and chronic inflammatory disorders and risk of non‐Hodgkin lymphoma by subtype. J Natl Cancer Inst. 2006;98(1):51‐60. [DOI] [PubMed] [Google Scholar]
- 12. Efthimiou P, Kontzias A, Hur P, Rodha K, Ramakrishna GS, Nakasato P. Adult‐onset Still's disease in focus: clinical manifestations, diagnosis, treatment, and unmet needs in the era of targeted therapies. Semin Arthritis Rheum. 2021;51(4):858‐874. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


