Abstract
The COVID-19 pandemic impacted the transmission of many pathogens. The aim was to determine the effect of non-pharmaceutical interventions on the incidence of diseases transmitted via food. Weekly incidence rates for nine foodborne pathogens were collected from national surveillance registries. Weekly pathogen incidence during lockdown weeks of 2020 and 2021 were compared with corresponding weeks in 2015–2019. The same analyses were performed to determine the effect of self-defined expected impact levels of measures (low, intermediate and high). Eight out of 9 diseases showed a significant decrease in case number in 2020, except for listeriosis, which remained unchanged. The largest decrease was observed for rotavirus gastronteritis A (−81%), norovirus gastroenteritis (−78%), hepatitis A (−75%) and shigellosis (−72). In 2021, lower case numbers were observed for 6 out of 9 diseases compared with 2015-2019, with the largest decrease for shigellosis (−5/%) and hepatitis E (−47%). No significant change was observed for listeriosis, STEC infection and rotavirus gastroenteritis. Overall, measures with increased expected impact level did not result in a larger decrease in number of cases, except for Campylobacter, and norovirus and rotavirus gastroenteritis. Disease transmitted via food significantly decreased during the COVID-19 pandemic, with a more pronounced effect during 2020 than 2021.
Keywords: COVID-19, foodborne infections, non-pharmaceutical interventions, surveillance, public health
Introduction
On 11 March 2020, the World Health Organization announced the pandemic of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In the Netherlands, extensive non-pharmaceutical interventions (NPIs) were taken to decrease the transmission of SARS-CoV-2, including local and international travel restrictions, lockdowns and stay-at-home recommendations, universal handwashing guidance, physical distancing, and face covering. These preventive measures did not only affect the spread of SARS-CoV-2, but also other infectious diseases. Most studies have focused on significant declines in other respiratory viruses, such as influenza, influenza-like illness, and pertussis, but decreases in several invasive diseases, vaccine preventable disease, and gastrointestinal diseases have also been reported [1–10]. Although the impact of NPIs was greatest for pathogens that (indirectly) spread from person to person, decreases have also been observed for pathogens associated with foodborne transmission.
Although the effect of NPIs on foodborne transmission might be less obvious, it could be related to measures such as closure of restaurants and increased hand hygiene. Likewise, there have been numerous reports on underdiagnosis and underreporting due to healthcare systems being under enormous pressure, as well as healthcare avoidance, that may have impacted the number of cases captured by surveillance [1, 5, 7–9]. Because the relative importance of foodborne transmission varies per pathogen, as well as the type of food products involved, differences in the impact NPIs had per pathogen are expected to vary. For example, previous studies found the impact to be largest on viral gastrointestinal infections, such as norovirus and rotavirus, compared with bacterial gastrointestinal infections such as Salmonella and Campylobacter [5, 9, 11, 12]. This is likely because the fraction of infections attributable to person-to-person transmission is much larger for norovirus and rotavirus than it is for Salmonella and Campylobacter [13].
The COVID-19 pandemic offers an opportunity to gain more insight into the drivers of gastrointestinal infections transmitted via food. The aim of this study was to determine the impact of the NPIs during COVID-19 pandemic on the incidence of bacterial and viral diseases that can be transmitted through food.
Methods
Data collection
Diseases were selected based on their ability to be transmitted via food and availability of national surveillance data during 2015–2021. These included five bacterial (campylobacteriosis, salmonellosis, shigellosis, Shiga toxin-producing Escherichia coli (STEC) infections, and listeriosis) and four viral diseases (hepatitis A, hepatitis E, norovirus, and rotavirus gastroenteritis).
Data on campylobacteriosis in the Netherlands were obtained from the Infectious Disease Surveillance Information System for Antibiotic Resistance (ISIS-AR), which collects data on antibiotic resistance from a large number of medical microbiology laboratories in the Netherlands. In 2021, it had an estimated nationwide coverage of 64%. Epidemiological data are limited to age, gender, date of sampling, and serotype. Data on salmonellosis are obtained via the national laboratory surveillance carried out by the National Institute for Public health and the Environment (RIVM), with an estimated coverage of 64% as well. Epidemiological data included age, gender, travelling abroad (reported in a subset of cases), date of sampling, and serotype.
Infections with Shigella, STEC, Listeria monocytogenes, and hepatitis A virus are notifiable in the Netherlands. Age, gender, report of travelling abroad, and date of illness onset and serotype (for Shigella) were extracted from the notifications. For STEC, Listeria monocytogenes, and hepatitis A virus, serotype information was obtained from the voluntary laboratory surveillance systems at the RIVM.
Data on hepatitis E, norovirus, and rotavirus were obtained from the Virological Laboratory Surveillance, consisting of approximately twenty virological laboratories that serve primary and/or secondary care, affiliated with the Dutch Working Group for Clinical Virology (NKWV) of the Dutch Association for Medical Microbiology (NVMM), that weekly report the aggregated number of detections of a large number of viral pathogens. These data do not contain information on gender, age, or travelling.
Measures
In the Netherlands, the first measures to control the spread of SARS-CoV-2 were implemented in week 11 of 2020 and the last measures were lifted in week 15 of 2022. We constructed a timeline by week where measures were categorised a priori according to their self-defined expected impact on daily life (0 = no measure or very low impact; 1 = some restrictions apply, but estimated impact is low; 2 = moderate impact; 3 = high impact/lockdown in place). The level of the lockdown measure was used as basis for defining the impact extended with the impact of the other measures (see Supplementary Table S1 and Supplementary Figure S1). Three binary variables were constructed, one for each defined impact level, where one were the weeks with that impact level, and 0 were the same weeks in 2015–2019. During the COVID-19 lockdown period, various containment measures such as stay-at-home order, travel restrictions, and business closures were often implemented concurrently. Hence, multicollinearity between measures hampered our ability to study the effect of individual measures, and we could only study the combined effect of measures.
Analyses
For each disease, we determined the association between the weekly disease incidence and the NPIs to control the spread of SARS-CoV-2. For this purpose, the incidence in the weeks of the measures during 2020 (week 11–52) and 2021 (week 1–52) were compared with the incidence in the same weeks during 2015–2019. Per disease, generalized linear models with Poisson distribution and log link function, or negative bionomical distribution in case of overdispersion, were used, with the weekly disease incidence as outcome and lockdown as binary dependent variable. Age (categorized into 0-4, 5-17, 18-39, 40-59, and 60+ years of age) and gender were always adjusted for. Data on population were included as offset variable to account for changes in demographics over the years and were obtained via Statistics Netherlands (www.cbs.nl). The same analyses were also performed by age groups and gender. Analyses by age groups were adjusted for gender and vice versa. Moreover, the analyses were repeated after exclusion of travel-related cases for diseases for which travel information was available and travel was reported as route of infection at least once during lockdown. These additional analyses were only possible for hepatitis A, listeriosis, salmonellosis, shigellosis, and STEC due to data availability. To determine whether there was a gradual decrease in number of cases with increasing impact of NPIs, a separate model was built for each of the impact score (i.e. low, moderate, and high) per disease. For six pathogens, separate models were used per subtypes to identify changes in number of cases by subtype: Listeria monocytogenes (serotypes 1/2a, 1/2b, 4b), Campylobacter (species jejuni and coli), STEC (serogroups O157, O26, and other), Shigella (species sonnei, flexneri), Salmonella (serotypes Enteritidis, Typhimurium, other), and hepatitis A virus (genotype I.A, I.B, III.A). For each of these analyses, separate models were made for 2020 and 2021, comparing the weeks in those years with the same weeks in 2015–2019. Furthermore, Pearson’s correlation coefficient was used to identify the lag with the strongest correlation between case count and the measures during 2020, which was the lag used for analyses. A lagged case count variable was entered in the model by shifting the values of the predictor variable by the number of weeks with the strongest correlation. The lag was restricted to a maximum of one week above the usual incubation period, with a maximum of four weeks (see Supplementary Table S2). Because there was a large international outbreak of hepatitis A among men who have sex with men (MSM) in 2017–2018 [14], cases related to this outbreak were excluded. Results are reported as exponentiated coefficients, subtracted by 1, with corresponding 95% confidence intervals (95% CI), which can be interpreted as the percentage increase or decrease in number of cases compared with 2015–2019. Analyses were performed in RStudio (version 2022.07.02).
Results
During the lockdown period in 2020, eight out of nine diseases showed a significant decrease in weekly disease incidence compared with 2015–2019 (Figures 1 and 2). The largest decrease was observed for rotavirus gastroenteritis (-81.4%, 95%CI: -87.5%, -72,5%), norovirus gastroenteritis (-78.0%, 95%CI: -82.7%, -72.0%), hepatitis A (-74.9%, 95%CI: -83.7%, -61.4%), and shigellosis (-72.0%, 95%CI: -77.0%, -65.9%). This was followed by salmonellosis (-53.4%, 95%CI: -58.9%, -47.3%), hepatitis E (-38.9%, 95%CI: -50.5%, -24.7%), campylobacteriosis (-34.8%, 95%CI: -38.9%, -30.4%), and STEC infection (-32.4%, 95%CI: -41.5%, -21.9%). The number of cases in 2020 only remained similar to 2015–2019 for listeriosis (+3.0%, 95%CI: -24.2%, +32.8%).
Figure 1.
Changes in disease incidence during weeks with measures in 2020 (week 11-52) and 2021 (week 1-52) compared with the same weeks in 2015-2019, including both domestically acquired and travel related cases.
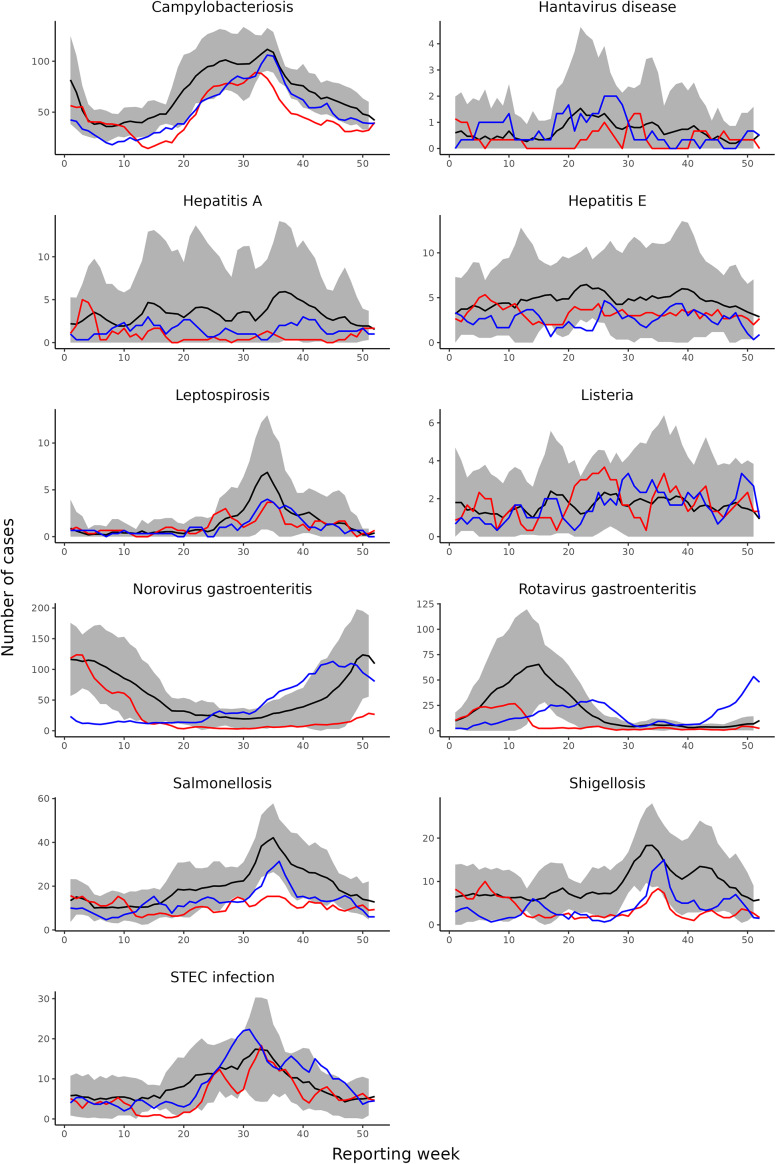
Figure 2.
Weekly case numbers using three-week moving averages by disease in 2015-2019 (black line,including 95% confidence interval), 2020 (red line), and 2021 (blue line).
In 2021, the number of cases was significantly lower than 2015–2019 for six out of nine diseases, but case numbers were overall higher than in 2020. The largest decrease was observed for shigellosis (-57.5%, 95%CI: -63.6%, -50.4%), followed by hepatitis E (-46.6%, 95%CI: -56.4%, -34.5%), salmonellosis (-34.9%, 95%CI: -41.5%, -27.6%), hepatitis A (28.9%, 95%CI: -45.1%, -8.0%), norovirus gastroenteritis (-27.9%, 95%CI: -41.9%, -10.5%), and campylobacteriosis (-25.9%, 95%CI: -30.2%, -21.3%). STEC infection (+4.2%, 95%CI: -7.5%, +17.4%), listeriosis (-10.2%, 95%CI: -31.1%, +17.0%), and rotavirus gastroenteritis (-18.9%, 95%CI: -40.3%, +10.1%) showed no significant decrease in number of reported cases in 2021.
Effect by age and gender
Overall, the median percentage decrease in incidence over all diseases was less prominent with increasing age. A median decrease of 40.7% was observed in age group 0-4 years, 38.8% decrease in age groups 5-17, 37.8% decrease in age group 18-39 years, 36.6% decrease in age group 40-59 years, and 36.2% decrease in those 60+ years old (Figure 3). However, the age group 0-4 years decreased less compared to other age groups in 2021 for Campylobacter and STEC when comparing to case numbers in 2015–2019. Females generally had a larger decrease in disease incidence compared with males, with a median decrease of 35.6% over all diseases compared with 33.3% in males (Supplementary Figure S2). This was most pronounced for shigellosis, with a 85.5% decrease in 2020 (95%CI: -78.2%, -90.3%) and 73.0% decrease in 2021 (95%CI: -63.9%, -79.8%) in females, compared with a decrease of 60.9% (95%CI: -51,3, -68.7) and 45.0% (95%CI: -34.3%, -53.9%), respectively, in males.
Figure 3.
Changes in disease incidences during the lockdown weeks in 2020 (week 11-52) and 2021 (week 1-52) compared with the same weeks in 2015-2019, by age groups, including both domestically acquired and travel related cases.
Effect on domestically acquired cases
To determine the effect that NPIs had on domestically acquired cases, travel-related cases were excluded from the analyses for five out of nine diseases for which information on travel was available. Overall, the decrease in total number of cases was larger than the decrease in domestically acquired cases only, although this difference was often not significant (Figure 4). Although the number of domestically acquired cases was still significantly lower in 2020 and 2021 compared with 2015-2019 for shigellosis, the decrease was significantly less pronounced.
Figure 4.
Changes in overall incidence and incidence of domestically acquired cases per disease during lockdown weeks in 2020 (week 11-52) and 2021 (week 1-52) compared to the same weeks in 2015-2019. The dark blue boxplots represents the percentage change in the overall disease incidence (travel and domestically acquired cases) and the light blue boxplots represents the percentage change in the disease incidence of domestically acquired cases.
Effect of impact level of measures
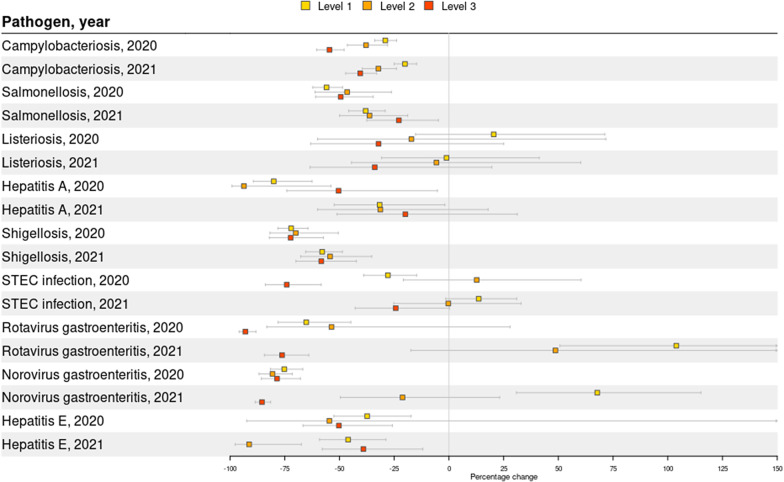
The expected effect of the measures on the number of cases was also assessed by levels of impact, with level 1 being mild expected impact and level 3 being high impact (i.e. lockdown). For campylobacteriosis, the number of cases in 2020 decreased -29.1% (95%CI: -23.9%, -33.9%) during level impact 1, -37.9% (95%CI: -28.0%, -46.4%) during level 2, and -54.6% (95%CI: -47.9%, 60.4%) during level 3 (Figure 5). In 2021, this was 20.1% (95%CI: -14.8%, -25.0%), 32.2% (95%CI: -24.0%, -39.6%), and 40.5% (95%CI: -33.0%, 47.2%), respectively. For rotavirus gastroenteritis, the number of cases increased +103.9% (95%CI: 50.7%, 175.9%) during level 1, +48.7% (95%CI: -17.4%, +167.5%) during level 2, and decreased -76.2% during level 3 (95%CI: -64.0%, -84.3%). For norovirus gastroenteritis, the number of cases increased +67.8% (95%CI: 30.8%, 115.2%) during level 1, and decreased -21.2% (95%CI: --49.6.4%, +23.2%) during level 2, and decreased -85.4% during level 3 (95%CI: -88.5%, -81.5%). Other diseases, however, had varying impact per level, without apparent larger effect of higher impact levels on the number of cases.
Figure 5.
Changes in disease incidence per expected impact level in 2020 and 2021 compared to 2015-2019. Level 1 was defined as a mild impact and level 3 as a high impact (lockdown).
Effect by subtype
For six pathogens, analysis was stratified by subtype. The number of Shigella sonnei cases had dropped significantly more in 2020 and 2021 than of Shigella flexneri (Supplementary Figure S3). When excluding travel-related cases, there was no significant decrease in number of domestically acquired Shigella flexneri cases in 2020 and even a significant increase in 2021. Finally, for hepatitis A, the number of cases only significantly decreased for type I.A in 2020 and only for the overall number of cases with type I.A in 2021, but not for the domestically acquired cases. No significant differences were seen between the most common serotypes of Campylobacter, Listeria monocytogenes, Salmonella, and STEC.
Discussion
During the COVID-19 pandemic, a continuum and variety of control measures were applied to prevent the spread of SARS-CoV-2. In addition to the intended effect – curbing the number of COVID-19 cases – it also affected a large number of other infectious diseases. Nine diseases that can be transmitted via the foodborne route were examined in the present study, including campylobacteriosis, hepatitis A, hepatitis E, listeriosis, norovirus gastroenteritis, rotavirus gastroenteritis, salmonellosis, shigellosis, and STEC infection. In 2020, the number of cases of all studied diseases except listeriosis had decreased. In 2021, the overall decrease in number of cases was less pronounced, with significantly lower case numbers for six out of nine diseases, including campylobacteriosis, hepatitis E, norovirus gastroenteritis, salmonellosis, and shigellosis. Overall, case numbers decreased most in the younger age groups.
The largest decrease in case numbers was observed for pathogens with a higher fraction of infections attributable to person-to-person transmission, including norovirus and rotavirus gastroenteritis, and Shigella, particularly during 2020, compared to pathogens mainly transmitted through food, such as Campylobacter and Salmonella [15]. We hypothesize that this could be because NPIs had differential impact depending on transmission route, mainly impacting person-to-person transmission due to increased emphasis on hand hygiene and physical distancing. Alternatively, travel restrictions, decreased dine-in options, and increased hygiene may have also contributed to decreased foodborne transmission. However, the reasons for changes in case numbers are likely to be multifactorial and may not reflect true changes in disease incidences. For example, we could not take into account changes in healthcare seeking behaviour and laboratory testing, which may have impacted the number of cases captured by surveillance [16]. Moreover, the impact may even be disease-specific, with a larger effect on disease with a milder course of the disease, such as norovirus for which patients may have refrained from seeking medical care, compared with campylobacteriosis and salmonellosis, which are more often associated with bloody stools. Findings in this study were largely consistent to previous studies reporting on the effect of NPIs on gastrointestinal and/or foodborne infections. For example, Germany reported a larger decrease in 2020 for rotavirus gastroenteritis, norovirus gastroenteritis, and shigellosis than for salmonellosis and campylobacteriosis, and similar findings were reported in Australia, England, Korea, Switzerland, and the United States [5, 9, 17–21]. Although most other studies have limited overlap in terms of foodborne infections included in our analyses, making comparison difficult, they generally also reported larger reductions for viral enteric diseases compared to bacterial enteric diseases [20, 22–25].
Among all age groups, the youngest (0-4 years) had the largest decrease in number of cases, which was also observed for a large range of notifiable diseases in Germany and only in the youngest age groups in England [5, 9]. Whether this is due to changes in healthcare utilization per age group or reflects true changes in number of cases is difficult to evidence. However, we hypothesize that cases were more likely to be diagnosed in the younger and older age groups compared to other age groups during the COVID-19 pandemic because of their vulnerability.
Listeriosis was the only disease with a stable number of cases during the COVID-19 pandemic compared to the years before. This suggests that the transmission route of Listeria monocytogenes, mainly pre-packaged, ready-to-eat food, stored and prepared without thorough heating at home, was not altered. An increase in underdiagnosis, underreporting, and healthcare avoidance may have had limited effect on listeriosis because of its severity almost always leading to hospitalization. Although similar findings were reported in Korea, Germany and the United States observed a significant decrease in the number of listeriosis cases in 2020; however, the number of listeriosis cases in the United States were back at pre-pandemic levels in 2021 [5, 10, 1 8, 26]. The reason for these differences between countries, however, is unknown.
After a substantial drop in the number of rotavirus cases in the Netherlands in 2020, the number of rotavirus cases did not significantly decrease in 2021 compared with pre-COVID-19 years. This was due to an early onset of the 2022 rotavirus season in October 2021, which was most likely the result of an accumulation of susceptible children that did not acquire a rotavirus infection during 2020 and 2021 rotavirus season. A similar phenomenon was observed for respiratory syncytial virus (RSV) in the Netherlands, for which a drop in antibody concentrations was observed during the COVID-19 pandemic, resulting in out-of-season RSV activity [27]. In Northern China, a rebound of rotavirus gastroenteritis was also observed after re-opening of schools in September 2020 [19].
Most of the reduction in shigellosis cases was due to a drop in travel-related cases. Subtype-specific analyses revealed that the number of domestically acquired Shigella flexneri cases was similar to pre-COVID-19 years in 2020 and even significantly higher in 2021. This was because the number of Shigella flexneri cases among MSM significantly increased in 2021 compared with pre-COVID-19 years [28]. This could also explain the larger decrease that was observed in females compared with males. The number of domestically acquired Shigella sonnei infections, however, decreased significantly during both pandemic years. For hepatitis A, a significant drop in the overall number of hepatitis A cases was observed, but not for the number of domestic cases. This could be because of two outbreaks in 2021: one with genotype I.A (14 cases) and one with I.B (7 cases). Because cases had not travelled and were geographically spread, the source was most likely a food product, but could not be confirmed.
Analyses on the different impact levels of measures showed that only the number of campylobacteriosis cases gradually decreased with an increasing impact of NPIs. A potential reason could be that the impact of the measures leads to shifts in transmission possibilities of the pathogen. For example, full closure of restaurants during the highest impact level facilitates spread of foodborne pathogens through take-away or delivery services. However, a similar effect would then be expected for Salmonella, which has relatively similar transmission routes compared with Campylobacter. Alternatively, the period of measures with the highest impact was generally during time periods when the incidence for most of the pathogens included in this study is usually low. Indeed, measures with the highest impact level were mainly in place during January to May, while the number of cases for many of the included diseases is highest during summer, including campylobacteriosis, shigellosis, and STEC infection. This likely also explains why the number of rotavirus and norovirus gastroenteritis cases only dropped significantly during measures with the highest impact level in 2021, because these measures were during the months of the usual norovirus and rotavirus season.
The effect of NPIs on case numbers was generally larger in 2020 than in 2021. A potential explanation could be that the compliance of the Dutch general population to the NPIs decreased throughout the course of the pandemic, or because there were different types or less stringent NPI’s [29]. Furthermore, healthcare avoidance, underdiagnosis, and underreporting may also have been more prevalent in 2020 compared to 2021, although this is difficult to [16]. Preliminary data from the United States report similar findings, with the incidence of foodborne diseases including campylobacteriosis, listeriosis, shigellosis, and STEC infection, increasing in 2021 compared with 2020, and sometimes even stabilizing or increasing compared with pre-COVID-19 years [18, 26]. However, they hypothesize that this may have been due to lifting of some of the pandemic control measures, while our analyses only focuses on weeks in 2021 where control measures were in place.
Our study has several limitations. First, we cannot infer causality of the impact of NPIs on pathogen circulation from the surveillance data. Based on our analyses, it is not possible to distinguish between the effect of NPIs and the effect of healthcare avoidance, underdiagnoses, and/or underreporting due to healthcare being overwhelmed and concerns around infection risks during healthcare visits. Second, most of the NPIs were implemented at the same time, making it impossible to separate the effect of, for example, restaurant closures from social distancing. Therefore, it was not possible to estimate measure-specific effects.
Concluding, the COVID-19 pandemic has had a major impact on foodborne infections in the Netherlands. However, disentangling the impact specific NPIs had on the effect of foodborne disease circulation from the effect of, for example, healthcare avoidance and testing strategies is challenging. Overall, the effect of the COVID-19 pandemic seems to have been largest for diseases with a lower fraction of cases being attributable to foodborne transmission. Moreover, case numbers were more heavily impacted in 2020 compared with 2021, with an upsurge in cases for rotavirus gastroenteritis at the end of 2021. The latter also highlights the importance of close monitoring of foodborne diseases as they may follow an untraditional seasonal pattern in coming years due to an accumulation of susceptible persons.
Supporting information
Pijnacker et al. supplementary material
Acknowledgements
The authors would like to thank participating laboratories of ISIS-AR for providing data on campylobacteriosis and laboratories participating in Virological Laboratory Surveillance for providing data on hepatitis E, norovirus, and rotavirus.
Supplementary material
For supplementary material accompanying this paper visit http://doi.org/10.1017/S0950268824000815.
click here to view supplementary material
Data availability statement
The data that support the findings of this study are available on request.
Author contribution
Conceptualization: I.H.F., L.V., E.F., L.M., R.P.; Data curation: I.H.F., L.M., M.v.d.B., R.P.; Methodology: I.H.F., L.V., E.F., L.M., R.P.; Project administration: I.H.F.; Supervision: I.H.F.; Writing – original draft: I.H.F., R.P.; Writing – review & editing: I.H.F., L.V., E.F., L.M., M.v.d.B.; Formal analysis: L.V., L.M., R.P.; Visualization: R.P.
Funding statement
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interest
The authors declare none.
References
- 1.Kuo SC, et al. (2020) Collateral benefit of COVID-19 control measures on influenza activity, Taiwan. Emerging Infectious Diseases 26, 1928–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen SJ, et al. (2020) Decreased influenza activity during the COVID-19 pandemic-United States, Australia, Chile, and South Africa, 2020. American Journal of Transplantation 20, 3681–3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soo RJJ, et al. (2020) Decreased influenza incidence under COVID-19 control measures, Singapore. Emerging Infectious Diseases 26, 1933–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan SG, et al. (2020) Where has all the influenza gone? The impact of COVID-19 on the circulation of influenza and other respiratory viruses, Australia, March to September. Euro Surveillance 2020, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ullrich A, et al. (2021) Impact of the COVID-19 pandemic and associated non-pharmaceutical interventions on other notifiable infectious diseases in Germany: An analysis of national surveillance data during week 1-2016 - week 32-2020. The Lancet Regional Health Europe 6, 100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huh K, et al. (2021) Impact of nonpharmaceutical interventions on the incidence of respiratory infections during the coronavirus disease 2019 (COVID-19) outbreak in Korea: A nationwide surveillance study. Clinical Infectious Diseases 72, e184–e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middeldorp M, et al. (2021) Short term impact of the COVID-19 pandemic on incidence of vaccine preventable diseases and participation in routine infant vaccinations in the Netherlands in the period March-September 2020. Vaccine 39, 1039–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicolay N, et al. (2020) Epidemiology of measles during the COVID-19 pandemic, a description of the surveillance data, 29 EU/EEA countries and the United Kingdom, January to May 2020. Euro Surveillance 25, 2001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Love NK, et al. (2022) Impact of the COVID-19 pandemic on gastrointestinal infection trends in England, February-July 2020. BMJ Open 12, e050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim S, et al. (2022) Trends in gastrointestinal infections before and during non-pharmaceutical interventions in Korea in comparison with the United States. Epidemiology and Health 44, e2022011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nachamkin I, et al. (2021) Reduction in sporadic norovirus infections following the start of the COVID-19 pandemic, 2019-2020, Philadelphia. Infectious Diseases and Therapy 10, 1793–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie O, et al. (2020) Physical distancing and non-respiratory notifiable diseases in the Northern Territory, March-May 2020. Communicable Diseases Intelligence (2018) 44, 1–6. [DOI] [PubMed] [Google Scholar]
- 13.Havelaar AH, et al. Attribution of foodborne pathogens using structured expert elicitation. Foodborne pathogens and disease. 2008;5:649–59. [DOI] [PubMed] [Google Scholar]
- 14.Ndumbi P, et al. (2018) Hepatitis A outbreak disproportionately affecting men who have sex with men (MSM) in the European Union and European Economic Area, June 2016 to May 2017. Euro Surveillance 23, 1700641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mughini-Gras L, et al. (2022) A statistical modelling approach for source attribution meta-analysis of sporadic infection with foodborne pathogens. Zoonoses Public Health 69, 475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Czeisler M, et al. (2020) Delay or avoidance of medical care because of COVID-19-related concerns - United States. MMWR Morbidity and Mortality Weekly Report 69, 1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steffen R, Lautenschlager S and Fehr J (2020) Travel restrictions and lockdown during the COVID-19 pandemic-impact on notified infectious diseases in Switzerland. Journal of Travel Medicine 27, taaa180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ray LC, et al. (2021) Decreased incidence of infections caused by pathogens transmitted commonly through food during the COVID-19 pandemic - Foodborne diseases active surveillance network, 10 U.S. Sites, 2017-2020. MMWR Morbidity and Mortality Weekly Report 70, 1332–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang LP, et al. (2021) The changing pattern of enteric pathogen infections in China during the COVID-19 pandemic: A nation-wide observational study. The Lancet Regional Health Western Pacific 16, 100268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn SY, et al. (2021) Changes in the occurrence of gastrointestinal infections after COVID-19 in Korea. Journal of Korean Medical Science 36, e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Love NK, et al. (2023) Understanding the impact of the COVID-19 pandemic response on GI infection surveillance trends in England, January 2020-April 2022. Epidemiology & Infection 151, e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bassal R, Keinan-Boker L and Cohen D (2021) A significant decrease in the incidence of Shigellosis in Israel during COVID-19 pandemic. International Journal of Environmental Research and Public Health 18, 3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mughini-Gras L, et al. (2021) Impact of the COVID-19 pandemic on human salmonellosis in the Netherlands. Epidemiology & Infection 149, e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen RT, et al. (2022) COVID-19 preventive measures coincided with a marked decline in other infectious diseases in Denmark, spring 2020. Epidemiology & Infection 150, e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Miguel Buckley R, et al. (2020) Social distancing to combat COVID-19 led to a marked decrease in food-borne infections and sexually transmitted diseases in Spain. Journal of Travel Medicine 27, taaa134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins JP, et al. (2022) Preliminary incidence and trends of infections caused by pathogens transmitted commonly through food - Foodborne diseases active surveillance network, 10 U.S. Sites, 2016-2021. MMWR Morbidity and Mortality Weekly Report 71, 1260–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.den Hartog G, et al. (2023) Decline of RSV-specific antibodies during the COVID-19 pandemic. The Lancet Infectious Diseases 23,23–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friesema I, et al. Annual report 2021 on enteric infections and zoonoses. Bilthoven; 2022.
- 29.Corona Gedragsunit (2021) Samenvatting onderzoeksresultaten Gedragsregels en Welbevinden: RIVM. Available at https://www.rivm.nl/gedragsonderzoek/maatregelen-welbevinden/samenvatting-onderzoeksresultaten.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pijnacker et al. supplementary material
For supplementary material accompanying this paper visit http://doi.org/10.1017/S0950268824000815.
click here to view supplementary material
Data Availability Statement
The data that support the findings of this study are available on request.