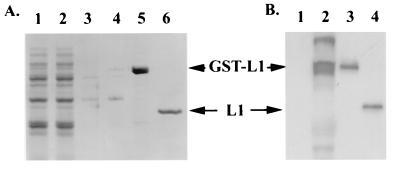
FIG. 1.
Purification of GST-COPV L1 protein after expression in E. coli. GST-COPV L1 protein was expressed and purified from bacteria by glutathione-Sepharose affinity chromatography. The indicated fractions were separated by SDS-PAGE and either stained with Coomassie blue (A) or immunoblotted with anti-L1 monoclonal antibody (B). (A) Lanes: 1, whole-cell lysate after IPTG induction; 2, flowthrough from glutathione-Sepharose column; 3, ATP-Mg2+ wash; 4, 2.5 M urea wash; 5, 10 mM reduced glutathione eluant; 6, eluant after thrombin digestion. (B) Lanes: 1, uninduced cell lysate; 2, cell lysate after induction with IPTG; 3, GST-L1 fraction; 4, L1 fraction after thrombin cleavage.