Abstract
Aim
Aging is a major cause of cognitive dysfunction. It has also been reported that respiratory function may influence cognitive dysfunction. However, few studies have examined the relationship between cognitive function and respiratory function among community‐dwelling older adults. This study aims to determine the relationship between respiratory function, assessed using spirometry, and mild cognitive impairment (MCI) in community‐dwelling older adults.
Methods
This study included 419 participants aged 73 ± 1 years and 348 participants aged 83 ± 1 years from the SONIC cohort study (Septuagenarians Octogenarians Nonagenarians Investigation with Centenarians Study). Respiratory function was evaluated using %Vital Capacity (%VC), Forced Expiratory Volume 1 s (FEV1)/Forced Vital Capacity (FVC), and %Peak Expiratory Flow (%PEF). Airflow‐limitation presence and stages were classified using FEV1/FVC. Cognitive function and MCI were assessed using the Japanese version of the Montreal Cognitive Assessment (MoCA‐J).
Results
The MoCA‐J score exhibited a declining trend as the airflow‐limitation stage increased among study participants in the 83 ± 1 age group. The presence of airflow limitation was associated with MCI in the 83 ± 1 age group. Among the indicators of each respiratory function, low %PEF was found to be associated with an increased rate of MCI. Furthermore, low %VC has also been suggested to be associated with an increased rate of MCI in the 83 ± 1 age female group.
Conclusions
Advanced airflow‐limitation stages may exacerbate cognitive dysfunction in community‐dwelling older adults. The presence of airflow limitation and low %VC may also be associated with cognitive dysfunction in older women. Consequently, reduced respiratory function may potentially be associated with MCI in community‐dwelling older adults. Geriatr Gerontol Int 2024; 24: 1001–1007.
Keywords: cognitive aging, epidemiology, pulmonary
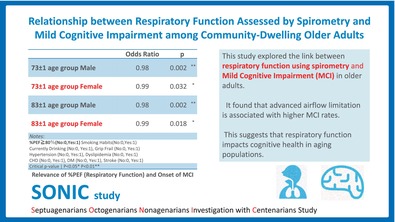
This study explored the link between respiratory function, assessed using spirometry, and mild cognitive impairment (MCI) in older adults. It found that advanced airflow limitation is associated with higher MCI rates. This suggests that respiratory function impacts cognitive health in aging populations.
Introduction
The number of dementia patients in the world is expected to reach 78 million by the end of this decade. 1 Screening systems and community‐based interventions for dementia prevention are currently being implemented in Japan. Although aging is a major cause of cognitive dysfunction, lifestyle‐related diseases, such as neurodegenerative diseases, diabetes, and hypertension, as well as lifestyle habits, such as smoking and heavy drinking, have also been reported as additional causes. 2
A relationship between respiratory function, which included airflow limitation as chronic obstructive pulmonary disease (COPD), and cognitive decline has recently been reported. 3 , 4 , 5 , 6 Given the aging population, COPD is anticipated to emerge as the third leading cause of death worldwide by 2030. 7 The significance of the relationship between respiratory and cognitive function is expected to rise. Respiratory and cognitive functions vary with age; hence, it is essential to investigate different age groups. However, the majority of previous studies have focused on young and mature adults, with limited research available on community‐dwelling older adults aged over 80 years.
Spirometry is the most commonly used instrument for assessing respiratory function. It measures the volume and speed of air that can be inhaled and exhaled. The spirometry test is noninvasive and can be conducted safely, making it suitable for use as a screening test. The examination of the relationship between cognitive and respiratory function indices has frequently involved the validation of %Vital Capacity (VC), %Forced Vital Capacity (FVC), and Forced Expiratory Volume 1 s (FEV1)/FVC. These indices fall within the category of ventilation impairment. Limited research has been conducted on %Peak Flow (PEF), which serves as an indicator of asthma.
In addition, respiratory ventilation requires respiratory movement, which involves the muscles that move the lungs and associated changes in pressure within the thoracic cavity. 8 Few studies have examined the effects of hypercapnic muscles and spirometry on respiratory function indices and cognitive function. Grip strength has been reported to be independently associated with maximal inspiratory and expiratory pressures in older adults. 9 Although respiratory muscle mass is difficult to measure, grip strength is used as an indicator of whole‐body muscle mass.
Therefore, the objective of this study is to investigate the correlation between respiratory and cognitive function, utilizing several respiratory function indicators and taking into consideration the frailty of grip strength. This study aims to determine the relationship between respiratory function, assessed using spirometry, and cognitive function in community‐dwelling older adults over 70 years of age.
Methods
Study participants
This cross‐sectional study drew from the ongoing SONIC study (Septuagenarians Octogenarians Nonagenarians Investigation with Centenarians Study), a prospective cohort initiated in 2010 with triennial follow‐ups. 10 Participants were drawn from two age groups: 72–74 years (73 ± 1 age group) and 82–84 years (83 ± 1 age group), who engaged in the SONIC study from 2013 to 2014. After excluding 31 individuals from the 73 ± 1 age group and 100 from the 83 ± 1 age group owing to missing data, outliers and individuals diagnosed with dementia, the final study cohort comprised 419 and 348 participants in the two groups, respectively. Previous studies have reported no significant association between Geriatric Depression Scale (GDS) scores, used to diagnose depression, and cognitive function. Therefore, participants with depression were not excluded from this study. 11 This study was approved by the Institutional Review Board of Osaka University Graduate School of Medicine, Dentistry, and Human Sciences (approval numbers: 266, H22‐E9, and 22 018, respectively). All the participants provided written informed consent to participate in this study.
Cognitive function
Cognitive function was assessed by trained professionals using the Japanese version of the Montreal Cognitive Assessment (MoCA‐J). The scores, ranging from 0 to 30, are adjusted for education years, with an additional point added for 12 years or less. 12 , 13 Higher scores reflect higher cognitive function. In comparison to conventional cognitive tests, MoCA‐J has demonstrated substantial reliability and validity for predicting early cognitive decline. 12 Mild cognitive impairment (MCI) was defined as an MoCA‐J score of 25 points or below. 13
Respiratory function
Trained medical personnel evaluated respiratory function using electronic diagnostic spirometry (SP‐370 COPD Pulmonary Per/Per Plus, Fukuda Denshi Ltd, Tokyo, Japan). The measurements of %VC (vs. standard lung capacity), %FVC (vs. standard forced lung capacity), %FEV1 (volume in 1 s), FEV1/FVC, and PEF (peak expiratory flow volume) were determined using the ratio of predicted values derived from age, sex, and height. These predicted values were based on normal benchmarks established by the Special Committee on Lung Physiology of the Japanese Respiratory Society. 14 Classification of ventilatory disturbances was based on the guidelines for respiratory function tests established by the Japan Respiratory Society. 15 Airflow‐limitation stages were defined based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) assessment tools to confirm the diagnosis of airflow limitation by spirometry. 7 The diagnosis of airflow limitation was defined as FEV1/FVC as measured by spirometry being less than 70%. 7 The stages of airflow limitation were defined as follows: Stage 1 for individuals with a %FEV1 of 80% or more, Stage 2 for those with %FEV1 between 50% and less than 80%, Stage 3 for %FEV1 between 30% and less than 50%, and Stage 4 for %FEV1 less than 30%. 7
Grip strength
Grip strength was measured using a hand dynamometer by trained physical therapists (Model YD‐100; Yagami Ltd, Nagoya, Japan). Participants were positioned in a seated posture, with their arms resting against the body. Two measurements were taken, and this study utilized the average of the first and second measurements. Weak grip strength was defined as <26 kg for men and < 18 kg for women. 16
Other factors
Medical history, lifestyle habits, and blood samples were obtained by medical professionals. Smoking habits were defined as current and past smoking. Stroke and cardiovascular heart disease (CHD) were diagnosed based on medical history. Hypertension was defined as positive if systolic blood pressure was ≥140 mmHg, diastolic blood pressure was ≥90 mmHg, or if the individual was undergoing antihypertensive treatment. 17 Diabetes mellitus (DM) was defined as positive if HbA1c was ≥6.5%, the casual blood glucose concentration was ≥200 mg/dL, or the individual was under medication for DM. 18 Dyslipidemia was defined as positive if non‐high‐density lipoprotein cholesterol was ≥170 mg/dL, high‐density lipoprotein cholesterol was <40 mg/dL, the triglyceride level was ≥150 mg/dL, or the individual was under medication for dyslipidemia. 19
Statistical analysis
Descriptive data are shown as a number (%) or medians (25% quartile–75% quartile). For descriptive statistics, the Mann–Whitney test was used for analysis. In the case of categorical variables, a chi‐square test was performed.
The Jonckheere–Terpstra test was used to compare the MoCA‐J scores among the airflow‐limitation stages. The relationships between respiratory function and MCI were examined using multiple logistic regression analysis including MCI as dependent variable, and sex, smoking habits, currently drinking, grip strength frail, hypertension, dyslipidemia, CHD, DM, and stroke as confounding factors. The presence of airflow limitation and indicators of each respiratory function were used as the independent variables. For analysis of indicators of each respiratory functions, model 1 included %VC, model 2 included FEV1/FVC, and model 3 included %PEF. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. All analyses were conducted using Statistical Package for the Social Sciences (SPSS) version 28 for Windows (IBM Japan, Tokyo, Japan), and the significance level was set at less than 5%.
Results
Characteristics of the study population
Table 1 summarizes the characteristics of the study population. In both age groups (73 ± 1 and 83 ± 1), approximately half of the participants were women. Comparing the two age groups, individuals in the 83 ± 1 group exhibited significantly lower height, weight, MoCA‐J scores, %PEF, and grip strength. Moreover, the prevalence of DM was higher in the 83 ± 1 age group than in the 73 ± 1 age group.
Table 1.
Characteristics of the study population
| 73 ± 1 age group | 83 ± 1 age group | P | |
|---|---|---|---|
| n = 419 | n = 348 | ||
| Female | 209 [50.1] | 169 [51.4] | 0.716 |
| Height (cm) | 157.7 [150.8–164.7] | 154.0 [154–160.4] | <0.01** |
| Weight (kg) | 57.3 [49.4–64.8] | 53.4 [45.6–60.3] | <0.01** |
| BMI (kg/m2) | 23.0 [20.7–24.9] | 22.4 [20.2–24.4] | 0.002** |
| Smoker (Number of People) | |||
| Current smoker | 60 [14.3] | 12 [2.9] | <0.01** |
| Past smoker | 110 [26.3] | 135 [32.2] | |
| No smoker | 249 [59.4] | 201 [48.0] | |
| Currently drinking (per) | 172 [41.1] | 121 [28.9] | 0.110 |
| Medical history (per) | |||
| Hypertension | 282 [67.3] | 268 [64.0] | 0.029* |
| Dyslipidemia | 177 [42.2] | 181 [43.2] | 0.036* |
| CHD | 81 [19.3] | 78 [18.6] | 0.123 |
| DM | 55 [13.1] | 87 [20.8] | <0.01** |
| Stroke | 33 [7.9] | 20 [4.8] | 0.351 |
| MoCA‐J (point) | 24.0 [22–26] | 21.4 [22–26] | <0.01** |
| GRIP (kg) | 25.2 [18.9–31.1] | 24.0 [19–31] | <0.01** |
| Respiratory function | |||
| %VC (%) | 142.5 [124.0–157.0] | 138.8 [155.2–155.2] | 0.066 |
| %FVC (%) | 132.3 [115.0–148.0] | 128.2 [108.5–150.4] | 0.097 |
| %FEV1 (%) | 132.0 [114.0–152.0] | 134.0 [111.3–160.7] | 0.169 |
| FEV1/FVC (%) | 79.2 [74.1–85.5] | 79.9 [75.1–87.3] | 0.117 |
| %PEF (%) | 107.1 [131.0–131.0] | 98.7 [67.7–128.3] | 0.003** |
| Air flow limitation (per) | |||
| Normal | 359 [85.7] | 298 [71.1] | 0.475 |
| Stage I | 49 [11.7] | 37 [8.8] | |
| Stage II | 10 [2.4] | 8 [1.9] | |
| Stage III | 1 [0.2] | 4 [1.0] | |
| Stage IV | 0 [0.0] | 1 [0.2] | |
| Ventilatory impairment (per) | |||
| Normal | 358 [85.4] | 290 [69.2] | 0.29 |
| Obstructive: FEV1/FVC ≦ 70% | 58 [13.8] | 49 [11.7] | |
| Restrictive: %VC ≦ 80% | 2 [0.5] | 5 [1.2] | |
| Mixed: FEV1/FVC ≦ 70%and %VC ≦ 80% | 1 [0.2] | 4 [1.0] |
Continuous variable, Mann Whitney‐test, median [IQR].
Categorical variable, χ2 test, number [%].
Critical P‐value, P < 0.05* P < 0.01**.
Abbreviations: CHD, coronary heart disease; DM, diabetes mellitus.
Relationship of airflow‐limitation stage with MoCA‐J
Figures 1 and 2 illustrate the relationship between MoCA‐J score and airflow‐limitation stage based on the GOLD classification across different age groups. Our analysis revealed a decline in MoCA‐J score with increasing airflow‐limitation stage especially within the 83 ± 1 age group.
Figure 1.
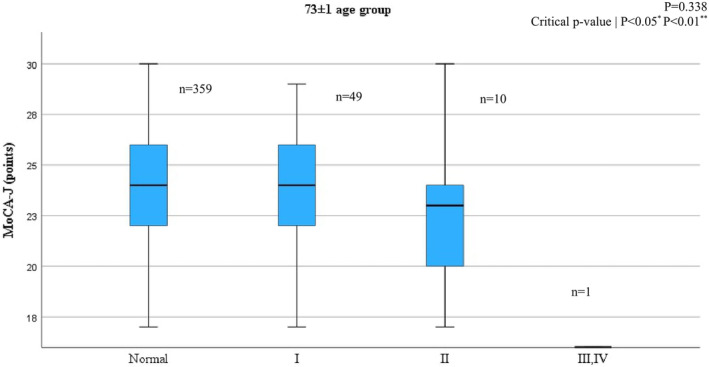
Relationship between airflow‐limitation stage and MoCA‐J score in the 73 ± 1 age group.
Figure 2.
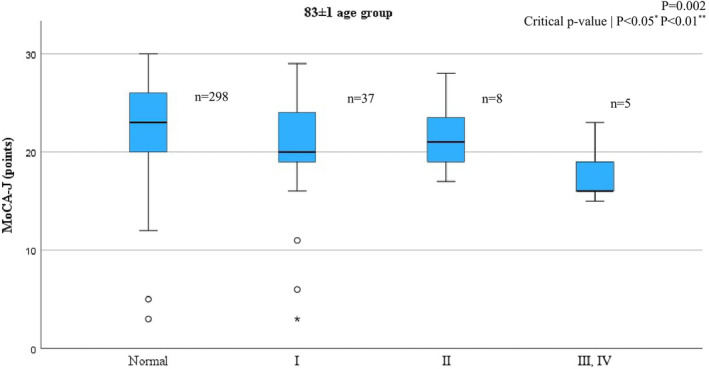
Relationship between airflow‐limitation stage and MoCA‐J score in the 83 ± 1 age group.
Factors associated with cognitive function
Table 2 presents the association between airflow limitation and MCI analyzed through multiple logistic regression in each age group. In the 73 ± 1 age group, females exhibited significantly higher cognitive function (P = 0.006). Conversely, in the 83 ± 1 age group, the presence of airflow limitation (P = 0.028, OR = 3.44, 95% CI = 1.141–10.340) and current drinking (P = 0.004, OR = 0.37, 95% CI = 0.189–0.734) were associated with lower cognitive function.
Table 2.
Factors associated with MCI
| Variables | 73 ± 1 age group | 83 ± 1 age group | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P | Odds ratio | 95% CI | P | |
| Gender (0: Male, 1: Female) | 2.45 | (1.290–4.643) | 0.006** | 1.59 | (0.715–3.543) | 0.255 |
| Smoking habit (0: No; 1: Current, Past) | 0.66 | (0.330–1.322) | 0.242 | 0.68 | (0.308–1.232) | 0.999 |
| Currently drinking (0: No; 1: Yes) | 1.21 | (0.703–2.087) | 0.491 | 0.37 | (0.189–0.734) | 0.004** |
| Grip frail (0: No; 1: Yes) | 1.35 | (0.741–2.452) | 0.329 | 1.42 | (0.683–2.937) | 0.349 |
| Hypertension (0: No; 1: Yes) | 0.93 | (0.567–1.540) | 0.790 | 0.69 | (0.338–1.392) | 0.296 |
| Dyslipidemia (0: No; 1: Yes) | 1.21 | (0.763–1.924) | 0.416 | 1.04 | (0.585–1.846) | 0.896 |
| CHD (0: No; 1: Yes) | 0.66 | (0.373–1.149) | 0.140 | 0.67 | (0.356–1.252) | 0.208 |
| DM (0: No; 1: Yes) | 1.10 | (0.566–2.136) | 0.779 | 1.89 | (0.906–3.935) | 0.090 |
| Stroke (0: No; 1: Yes) | 0.61 | (0.252–1.471) | 0.271 | 1.23 | (0.372–4.080) | 0.733 |
| Airflow limitation (0: No; 1: Yes) | 0.91 | (0.476–1.730) | 0.768 | 3.44 | (1.141–10.340) | 0.028* |
Critical P‐value: P < 0.05*, P < 0.01**.
Abbreviations: CHD, coronary heart disease; DM, diabetes mellitus.
Respiratory function test index and MoCA‐J
Table 3 displays the results of multiple logistic regression models examining the association between respiratory function test indices (%VC, FEV1/FVC, %PEF) and the presence of MCI in each age group. Across both age groups, a higher %PEF was associated with the presence of MCI after adjusting for covariates. (Male 73 ± 1 age, P = 0.002, ORs = 0.98, 95% CI = 0.974–0.994; Female 73 ± 1 age, P = 0.032, ORs = 0.99, 95% CI = 0.981–0.999; Male 83 ± 1 age, P = 0.002, ORs = 0.98, 95% CI = 0.969–0.993; Female 83 ± 1 age, P = 0.018, ORs = 0.99, 95% CI = 0.975–0.998). In the female group of age 83 ± 1, a higher %VC was associated with the presence of MCI (P = 0.011, ORs = 0.98, 95% CI = 0.974–0.994).
Table 3.
Respiratory function test index and MCI
| Odds ratio | 95% CI | P | ||
|---|---|---|---|---|
| 73 ± 1 age group | Model 1 | 1.00 | (0.990–1.014) | 0.728 |
| Male | Model 2 | 0.99 | (0.956–1.019) | 0.416 |
| Model 3 | 0.98 | (0.974–0.994) | 0.002** | |
| 73 ± 1 age group | Model 1 | 0.99 | (0.985–1.002) | 0.126 |
| Female | Model 2 | 0.99 | (0.957–1.024) | 0.561 |
| Model 3 | 0.99 | (0.981–0.999) | 0.032* | |
| 83 ± 1 age group | Model 1 | 0.99 | (0.980–1.006) | 0.269 |
| Male | Model 2 | 0.96 | (0.926–1.003) | 0.067 |
| Model 3 | 0.98 | (0.969–0.993) | 0.002** | |
| 83 ± 1 age group | Model 1 | 0.98 | (0.966–0.996) | 0.011* |
| Female | Model 2 | 0.99 | (0.956–1.023) | 0.527 |
| Model 3 | 0.99 | (0.975–0.998) | 0.018* |
Model 1: %VC ≧ 80% (No: 0; Yes:1), Smoking habits (No: 0; Yes: 1), Currently drinking (No: 0; Yes: 1), Grip frail (No: 0; Yes: 1), Hypertension (No: 0; Yes: 1), Dyslipidemia (No: 0; Yes: 1), CHD (No: 0; Yes: 1), DM (No: 0; Yes: 1), Stroke (No: 0; Yes: 1).
Model 2: FEV1/FVC ≧ 70% (No: 0; Yes: 1), Smoking habits (No: 0; Yes: 1), Currently drinking (No: 0; Yes: 1), Grip frail (No: 0; Yes: 1), Hypertension (No: 0; Yes: 1), Dyslipidemia (No: 0; Yes: 1), CHD (No: 0; Yes: 1), DM (No: 0; Yes: 1), Stroke (No: 0; Yes: 1).
Model 3: %PEF ≧ 80% (No: 0; Yes: 1), Smoking habits (No: 0; Yes:1), Currently drinking (No: 0; Yes: 1), Grip frail (No: 0; Yes: 1), Hypertension (No: 0; Yes: 1), Dyslipidemia (No: 0; Yes: 1), CHD (No: 0; Yes: 1), DM (No: 0; Yes: 1), Stroke (No: 0; Yes: 1).
Critical P‐value: P < 0.05*, P < 0.01**.
Discussion
This study investigated the correlation between spirometry‐assessed respiratory function and MCI in older adults aged 73 ± 1 and 83 ± 1. %PEF showed an independent association with MCI across both groups. A multiple regression analysis was conducted to examine the association between the MoCA‐J score, an indicator of cognitive function, and respiratory function indicators in a group with normal respiratory function (%VC and %PEF above 80%). The results showed a significant association between decreased respiratory function and decreased cognitive function in the group of women in the 73 ± 1 female group (data not shown).
In the 83 ± 1 female group, %VC was also linked to MCI, while FEV1/FVC was not. These findings align with prior research. 20 , 21
Previous studies have shown that airflow limitation has the greatest impact on cognitive function, which may be one of the reasons for our result. 20 This could be attributed to the %PEF being a more robust indicator of lung capacity than the FEV1/FVC ratio. Spirometry‐based respiratory indices reflect bronchial and respiratory muscle status. 22 Numerous reports in the existing literature have stated the correlation between grip strength and cognitive function. Cognitive function is closely linked to muscle strength, and muscle strength, in turn, is associated with respiratory function. Therefore, a comprehensive examination that considers grip strength while scrutinizing cognitive and respiratory functions is crucial. 23 However, few studies consider grip strength as a confounder. Our results imply that bronchial obstruction, rather than respiratory muscle function, may influence cognitive function in older adults.
Furthermore, there are limited community‐based studies on older adults in Japan, and it is worth noting that respiratory function can exhibit significant variations among different racial groups. 24 Further evidence regarding the relationship between respiratory and cognitive function in Japanese studies are required.
Our findings indicate that cognitive function tends to deteriorate in those who are in airflow‐limitation stages and currently drinking in the 83 ± 1 age group, in contrast to those without the condition. These results align with those of previous studies suggesting that airflow‐limitation worsening and aging may affect cognitive function 19 , 25 However, previous studies have reported an association between the airflow‐limitation stage worsening and cognitive function, even in younger and mature age groups. 3 Our findings differ from those of previous studies, in that we observed a significant association between airflow limitation and cognitive function exclusively in the 83 ± 1 age group. The mechanism by which airflow limitation impairs cognitive function is neuronal damage and depletion of neurotransmitters caused by hypoxia. 26 , 27 , 28 In addition, the inflammatory response, such as serum CRP and hs‐CRP etc, triggered by chronic respiratory disease and the cause of airflow limitation, has been reported to exacerbate cognitive decline. 29 , 30 , 31 Furthermore, aging accelerates the deterioration of cognitive function owing to atrophy and the aging of brain cells and cranial nerves, and aging with airflow limitation further deteriorates cognitive function. The absence of an association between airflow limitation and cognitive function in the 73 ± 1 age group in this study may be attributed to the fact that the participants included a healthy population living in the community who were able to participate in health surveys. Previous studies on the association between drinking habits and cognitive function have suggested that drinking habits contribute to cognitive decline, while other conflicting reports indicate the opposite. 32 , 33 The participants in this study were relatively healthy older adults in the community, which may have introduced survival bias and sample bias that may have influenced our results.
According to the Surgeon General's Report on Public Health in the United States (2004), 34 smoking causes acute respiratory diseases, including pneumonia. The mechanisms involved are not yet fully understood; however, it has been suggested that nicotine itself may inhibit the division of T cells, leading to changes in cytokine states that make individuals more prone to inflammation and susceptible to infections. In chronic respiratory diseases, smoking can disrupt the balance between oxidative stress, inflammation, and protein degradation regulation, causing physiological responses that impair the airways and alveoli in lung tissues.
Smoking is widely recognized as a risk factor for dementia in older adults. 35 Despite this, our multiple logistic analysis, which accounted for current and past smoking, did not reveal a significant association with MCI. However, evidence concerning community‐dwelling older adults remains insufficient, and while there are suggestions of a causal link between smoking and cognitive function, a conclusive correlation is yet to be established. 36 , 37 Therefore, our study contributes meaningfully to accumulating evidence on this relationship.
Several limitations should be noted. First, spirometry tests require participants' understanding and cooperation, posing challenges for cognitively impaired individuals or those with depressive moods, potentially introducing measurement bias. Therefore, individuals diagnosed with dementia were excluded from the statistical analysis in this study. Furthermore, a previous study on the impact of depressed mood on the reliability of spirometry tests revealed no significant correlation between GDS scores and spirometry results in older adults. 11 However, as this is a cross‐sectional study with outcomes defined as the onset of MCI or MoCA‐J scores, causality cannot be fully established.
Second, the diagnostic criteria of the airflow limitation rely solely on FEV1/FVC. Consequently, other COPD diagnostic criteria such as a long history of smoking, other respiratory diseases, and bronchodilator administration are not taken into consideration. Third, our study population consisted mainly of relatively healthy community‐dwelling older adults, potentially introducing survival bias. Fourth, previous studies have suggested that a longer period of non‐smoking is associated with a lower risk of cognitive decline. 38 However, it was not possible to consider the effects of the number of years of cigarette smoking. Finally, as a cross‐sectional study, causal relationships were not discernible, highlighting the need for longitudinal analyses in future research.
Conclusions
Our findings suggest that %PEF may exacerbate cognitive dysfunction in community‐dwelling older adults. The presence of airflow limitation and low %VC may also be associated with cognitive dysfunction in older women. In particular, the %PEF was the strongest indicator of MCI development in this study, which is a novel finding. The %PEF can be measured with a peak‐flow meter. The peak‐flow meter is simpler than a spirometer and can be used at home. Studies on the %PEF index may be useful as an indicator of cognitive and respiratory function decline in older individuals. Older adults have low immunity and are prone to respiratory infections that can aggravate their respiratory function. Preventing a decline in respiratory function is important for maintaining cognitive function.
In terms of the clinical application of this study, it is crucial to incorporate smoking cessation interventions, the impact of smoking on respiratory function until midlife being generally recognized, into health guidance aimed at maintaining cognitive function in older adults. However, there was no significant association between smoking habits and cognitive decline in the present study. Regardless of smoking habits, the results of this study indicate that airflow limitation can lead to cognitive decline in old age. Therefore, it is necessary to integrate respiratory function training and exercise guidance that can maintain respiratory function into health counseling programs designed to preserve cognitive function in older adults.
Disclosure statement
We declare no conflict of interest.
Author contributions
KK, YG, KI, and HR designed the study. KK, HR, MK, KG, YT, HA, KS, YT, YT, KY, MK, and MH collected medical data. YG and SY collected cognitive, psychological, and social data. MH conducted statistical analyses. MH, MK, and KK wrote the manuscript. All authors have reviewed and approved the final version.
Acknowledgements
We sincerely appreciate the cooperation of all SONIC study participants. Special thanks to the relevant members of Osaka University Graduate School staff, particularly Tomoko Noma, Hirochika Ryuno, Masaki Isaka, Toshiki Mizuno, Tomoko Yano, Arisa Wada, Yuko Nakamura, Hiroko Yoshida, Yuka Ohata, Naoko Murakami, Mio Kubo, Haruna Kikuchi, Yuka Fukata, Yuka Yokoyama, Riko Kinjo, Chihiro Morioka, Natsumi Fujiwara, Ayaka Hiratsuka, Kaoru Hatta, Fang Wen, Maya Mitani, Yui Toshimitsu, Chisato Hori, and Rena Yokokawa. This study received partial support from research grants, including Japan Society for the Promotion of Science KAKENHI 19K11138 (MK), 19K07888 (KK), A22Z034140 (MK), Ministry of Health, Labor and Welfare Research on Policy Planning and Evaluation Program (JPMH23AA2006), Osaka University's International Joint Research Promotion Program Support Type A (KK), and Support for Pioneering Research Initiated by the Next Generation, J219713005 (KK, YT).
Tachibana Y, Godai K, Kabayama M, et al. Relationship between respiratory function assessed by spirometry and mild cognitive impairment among community‐dwelling older adults. Geriatr. Gerontol. Int. 2024;24:1001–1007. 10.1111/ggi.14962
Data Availability Statement
Research data are not shared.
References
- 1. World Health Organization . A blueprint for dementia research, 2022. Available from https://apps.who.int/iris/handle/10665/363341.
- 2. Livingston G, Sommerlad A, Orgeta V et al. Dementia prevention, intervention, and care. Lancet 2017; 390: 2673–2734. [DOI] [PubMed] [Google Scholar]
- 3. Schou L, Ostergaard B, Rasmussen L et al. Cognitive dysfunction in patients with chronic obstructive pulmonary disease—a systematic review. Respir Med 2012; 106: 1071–1081. [DOI] [PubMed] [Google Scholar]
- 4. Simargi Y, Mansyur M, Turana Y et al. Risk of developing cognitive impairment on patients with chronic obstructive pulmonary disease a systematic review. Medicine 2022; 101: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang Y, Li X, Wei B, Tung T, Tao P, Chien C. Association between chronic obstructive pulmonary disease and dementia: systematic review and meta‐analysis of cohort studies. Dement Geriatr Cogn Disord Extra 2019; 9: 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yohannes A, Chen W, Moga A, Leroi I, Connolly M. Cognitive impairment in chronic obstructive pulmonary disease and chronic heart failure: a systematic review and meta‐analysis of observational studies. J Am Med Inform Assoc 2017; 18: 11–451.e11. [DOI] [PubMed] [Google Scholar]
- 7. Global Initiative for Chronic Obstructive Lung Disease Pocket Guide to COPD Diagnosis, Management and Prevention, 2023.
- 8. Roussos C, Macklem P. The respiratory muscles. N Engl J Med 1982; 307: 786–797. [DOI] [PubMed] [Google Scholar]
- 9. Bahat G, Tufan A, Ozkaya H et al. Relation between hand grip strength, respiratory muscle strength and spirometric measures in male nursing home residents. Aging Male 2014; 17: 136–140. [DOI] [PubMed] [Google Scholar]
- 10. Gondo Y, Masui Y, Kamide IK, Arai Y, Ishizaki T, SONIC study . A Longitudinal Cohort Study of the Older People as Part of a Centenarian Study; Encyclopedia of Geropsychology. In: SONIC Study: A Longitudinal Cohort Study of the Older People as Part of a Centenarian Study. Singapore: Springer Science + Business Media, 2017; 2227–2236. 10.1007/978-981-287-080-3_182-1. [DOI] [Google Scholar]
- 11. Pezzoli L, Giardini G, Consonni S et al. Quality of spirometric performance in older people. Age Ageing 2003; 32: 43–46. [DOI] [PubMed] [Google Scholar]
- 12. Fujiwara Y, Suzuki H, Yasunaga M et al. Brief screening tool for mild cognitive impairment in older Japanese: validation of the Japanese version of the Montreal cognitive assessment. Geriatr Gerontol Int 2010; 10: 225–232. [DOI] [PubMed] [Google Scholar]
- 13. Nasreddine Z, Phillips A, Bedirian V et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–699. [DOI] [PubMed] [Google Scholar]
- 14. Japan respiratory society special committee on lung physiology. Spyrogram and arterial blood gas partial pressure reference values for Japanese. JRS, 2001.
- 15. Japan respiratory society special committee on lung physiology. guidelines for respiratory function tests‐spirometry, flow volume curves, and pulmonary diffusion capacity, JRS, 2004. [PubMed]
- 16. Satake S, Shimada H, Yamada M et al. Prevalence of frailty among community‐dwellers and outpatients in Japan as defined by the Japanese version of the cardiovascular health study criteria. Geriatr Gerontol Int 2017; 17: 2629–2634. [DOI] [PubMed] [Google Scholar]
- 17. Japanese Society of Hypertension . Guidelines for the management of hypertension. Hypertens Res 2019; 42: 1235–1481. [DOI] [PubMed] [Google Scholar]
- 18. Araki E, Goto A, Kondo T et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int 2020; 11: 165–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kinoshita M, Yokote K, Arai H et al. Japan atherosclerosis society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb 2018; 25: 846–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vasilopoulos T, Kremen WS, Grant MD et al. Individual differences in cognitive ability at age 20 predict pulmonary function 35 years later. Epidemiol Community Health 2015; 69: 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vasilopoulos T, Grant MD, Franz CE et al. Shared and distinct genetic influences among different measures of pulmonary function. Behav Genet 2013; 43: 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Standardization of spirometry 1994 UPDATE. Am J Respir Crit Care 1995; 152: 1107–1136. [DOI] [PubMed] [Google Scholar]
- 23. Yang J, Deng Y, Yan H et al. Association between grip strength and cognitive function in US older adults of NHANES 2011‐2014. J Alzheimers Dis 2022; 89: 427–436. [DOI] [PubMed] [Google Scholar]
- 24. Pellegrino R, Viegi G, Brusasco V et al. Interpretative strategies for lung function tests. Rev Mal Respir 2007; 24: S83–S108. [Google Scholar]
- 25. Lung‐Function testing selection of refence values and interpretative strategies. Am Rev Respir Dis 1991; 144: 1202–1218. [DOI] [PubMed] [Google Scholar]
- 26. Lahousse L, Tiemeier H, Ikram M, Brusselle G. Chronic obstructive pulmonary disease and cerebrovascular disease: a comprehensive review. Respir Med 2015; 109: 1371–1380. [DOI] [PubMed] [Google Scholar]
- 27. Agusti A, Soriano JB. COPD as a systemic disease. J Chronic Dis 2008; 5: 133–138. [DOI] [PubMed] [Google Scholar]
- 28. Antonelli‐Incalzi R, Corsonello A, Trojano L et al. Correlation between cognitive impairment and dependence in hypoxemic COPD. J Clin Exp Neuropsychol 2008; 30: 141–150. [DOI] [PubMed] [Google Scholar]
- 29. Caramori G, Pandit A, Papi A. Is there a difference between chronic airway inflammation in chronic severe asthma and chronic obstructive pulmonary disease? Curr Opin Allergy Clin Immunol 2005; 5: 77–83. [DOI] [PubMed] [Google Scholar]
- 30. Maeda S, Takeya Y, Oguro R et al. Serum albumin/globulin ratio is associated with cognitive function in community‐dwelling older people: the septuagenarians, octogenarians, nonagenarians investigation with centenarians study. Geriatr Gerontol Int 2019; 19: 967–971. [DOI] [PubMed] [Google Scholar]
- 31. Hosokawa M, Kabayama M, Godai K et al. Association between high‐sensitivity C‐reactive protein and cognitive function in community‐dwelling older people. JPN J Geriatr 2024; 61: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Akagi Y, Kabayama M, Gondo Y et al. Alcohol drinking patterns have a positive association with cognitive function among older people: a cross‐sectional study. BMC Geriatr 2022; 22: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gutwinski S, Schreiter S, Priller J, Henssler J, Wiers C, Heinz A. Drink and think: impact of alcohol on cognitive functions and dementia—evidence of dose‐related effects. Pharmacopsychiatry 2018; 51: 136–143. [DOI] [PubMed] [Google Scholar]
- 34. A Report of the Surgeon General. USA Surgeon General Report, 2004. Available from; https://apps.who.int/iris/handle/10665/363341.
- 35. Reitz C, Den Heijer T, Van Duijn C, Hofman A, Breteler M. Relation between smoking and risk of dementia and Alzheimer disease—the Rotterdam study. Neurology 2007; 69: 998–1005. [DOI] [PubMed] [Google Scholar]
- 36. Hirayama T. Large cohort study on the relation between cigarette smoking and senile dementia without cerebrovascular lesions. Tob Control 1992; 1: 176–179. [Google Scholar]
- 37. Ikeda A, Yamagishi K, Tanigawa T et al. Cigarette smoking and risk of disabling dementia in a Japanese rural community: a nested case‐control study. Cerebrovasc Dis 2008; 25: 324–331. [DOI] [PubMed] [Google Scholar]
- 38. Galanis D, Petrovitch H, Launer L, Harris T, Foley D, White L. Smoking history in middle age and subsequent cognitive performance in elderly Japanese‐American men—the Honolulu‐Asia aging study. Am J Epidemiol 1997; 145: 507–515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.


