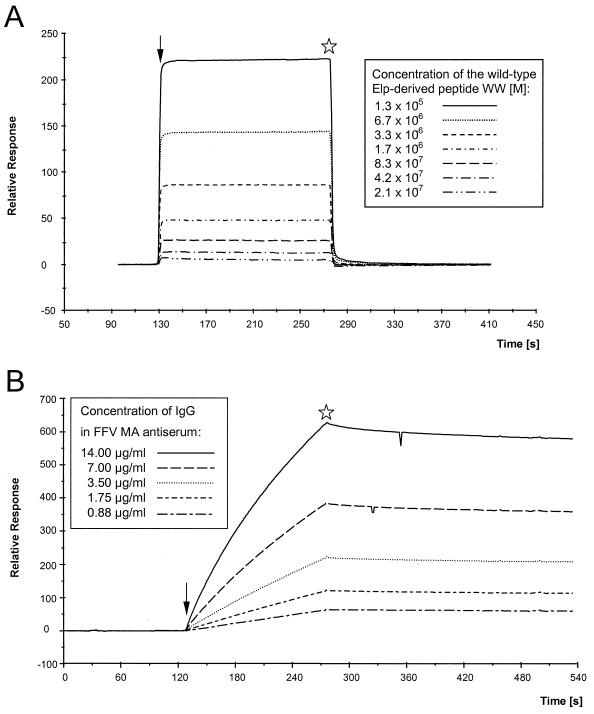
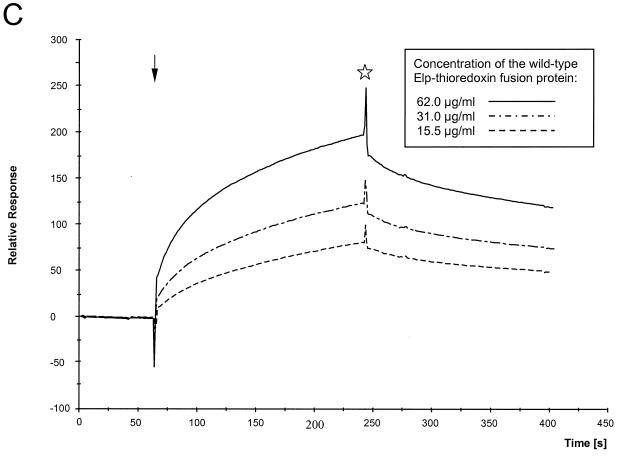
FIG. 8.
(A) Kinetic analyses of the interaction of the authentic Elp-derived peptide with FFV Gag 1–154 coupled to the CM5 sensor. The peptide WW was passed over the sensor chip at a flow rate of 10 μl/min for 2.5 min at concentrations ranging from 2.08 × 10−7 to 1.33 × 10−5 M as shown in the inset. The fast associations and dissociations are graphically expressed as relative response over time in minutes. Regeneration of the sensor surface was complete and achieved by changing to HBS buffer. Arrow, start of the binding reaction; asterisk, beginning of the washing with HBS buffer. (B) Kinetic analyses of the interaction of the FFV MA antiserum with FFV Gag 1–154 coupled to the CM5 sensor. Defined dilutions of the FFV MA antisera ranging from 1:500 to 1:8,000 were passed over the sensor chip at a flow rate of 10 μl/min for 2.5 min corresponding to immunoglobulin G concentrations from 14 to 0.88 μg/ml as shown in the inset. The slow association of the polyclonal antiserum at concentrations reaching saturation and the very low dissociation of the bound antibodies are graphically expressed as relative response over time in minutes. (C) Kinetic analyses of the interaction of the FFV Gag 1–154 protein coupled to the CM5 sensor with the purified thioredoxin-Elp 1–65 fusion protein. Defined concentrations of the recombinant thioredoxin-Elp 1–65 fusion protein ranging from 62 to 15.5 μg/ml were passed over the sensor chip at a flow of 15 μl/min for 3 min. The slow and specific association of the thioredoxin-Elp 1–65 and the slow dissociation of the bound Elp protein are graphically expressed as relative response over time in minutes. The thioredoxin protein without the FFV Elp domain did not show any specific binding. Arrow, start of the binding reaction; asterisk, beginning of the washing with HBS buffer.