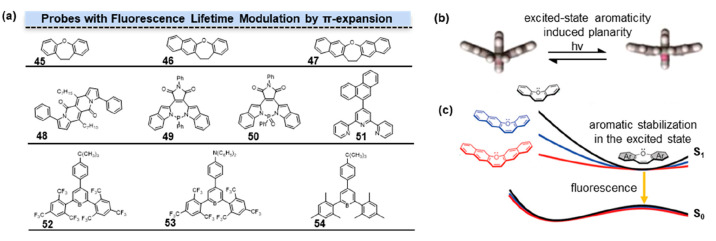
Figure 7.
Fluorescence lifetime modulation by π-expansion. (a) Structures of probes with fluorescence lifetime modulation by π-expansion. The degree of π-conjugation progressively increased from 45 to 47. Phenyl groups at the third and ninth positions extended the π-conjugation of DPND in 48. Different P-heteropines in π-systems had influences on fluorescence lifetime of 49 and 50. π-Expansion affected the conformation changes in the excited state of terpyridines (51) and triarylborane derivatives (52–54). (b) Excited-state aromaticity made the conformation tend to be planar. (c) Aromatic stabilization in the excited state. (b) Reprinted with permission from ref (36). Copyright 2023, American Chemical Society. (c) Reprinted with permission from ref (33). Copyright 2020, American Chemical Society.