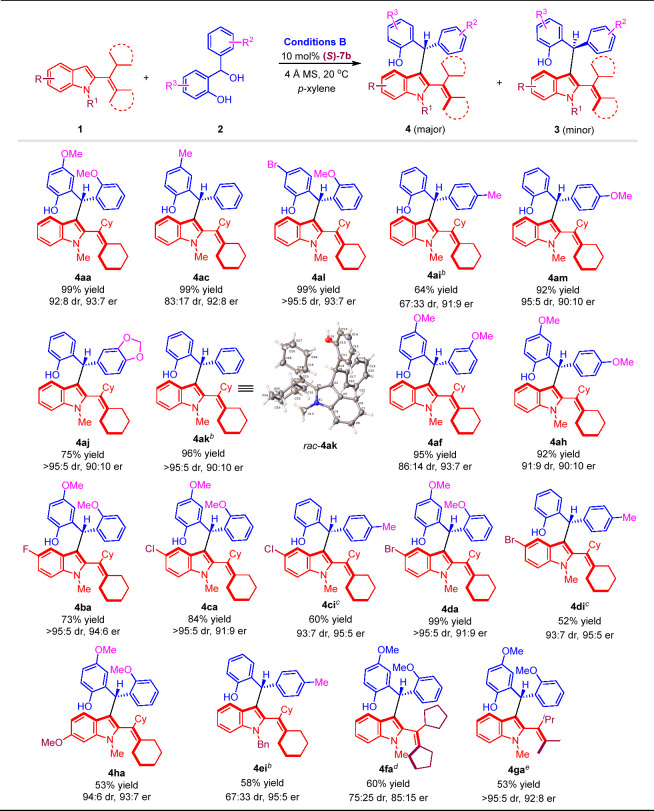
Table 2. Substrate Scope for Atroposelective Synthesis of Chiral 2-Alkenylindoles 4a.
Reaction conditions: 1 (0.2 mmol), 2 (0.1 mmol), (S)-7b (10 mol %), 4 Å MS (100 mg), p-xylene (4 mL), 20 °C for 12 h. Isolated yields were provided, the er value was determined by HPLC and the dr value (4:3) was determined by 1H NMR. The structure of rac-4ak was confirmed by single-crystal X-ray diffraction analysis,142 and the absolute configuration of 4ak was determined to be (Ra, R) by comparison with a known chiral compound (see the Supporting Information for details).
Catalyzed by 10 mol % (S)-7c.
Catalyzed by 10 mol % (S)-7c at −30 °C in mesitylene for 24 h.
Catalyzed by 10 mol % (R)-6b.
Catalyzed by 20 mol % (R)-6b at 0 °C in mesitylene for 24 h, 1g:2a = 3:1.