Abstract
GB virus B (GBV-B) is the closest relative of hepatitis C virus (HCV) and is an attractive surrogate model for HCV antiviral studies. GBV-B induces an acute, resolving hepatitis in tamarins. Utilizing primary cultures of tamarin hepatocytes, we have previously developed a tissue culture system that exhibits high levels of GBV-B replication. In this report, we have extended the utility of this system for testing antiviral compounds. Treatment with human interferon provided only a marginal antiviral effect, while poly(I-C) yielded >3 and 4 log units of reduction of cell-associated and secreted viral RNA, respectively. Interestingly, treatment of GBV-B-infected hepatocytes with ribavirin resulted in an approximately 4-log decrease in viral RNA levels. Guanosine blocked the antiviral effect of ribavirin, suggesting that inhibition of IMP dehydrogenase (IMPDH) and reduction of intracellular GTP levels were essential for the antiviral effect. However, mycophenolic acid, another IMPDH inhibitor, had no antiviral effect. Virions harvested from ribavirin-treated cultures exhibited a dramatically reduced specific infectivity. These data suggest that incorporation of ribavirin triphosphate induces error-prone replication with concomitant reduction in infectivity and that reduction of GTP pools may be required for incorporation of ribavirin triphosphate. In contrast to the in vitro studies, no significant reduction in viremia was observed in vivo following treatment of tamarins with ribavirin during acute infection with GBV-B. These findings are consistent with the observation that ribavirin monotherapy for HCV infection decreases liver disease without a significant reduction in viremia. Our data suggest that nucleoside analogues that induce error-prone replication could be an attractive approach for the treatment of HCV infection if administered at sufficient levels to result in efficient incorporation by the viral polymerase.
The original GB virus (GBV) inoculum was obtained from a surgeon with the initials G. B. who contracted non-A non-B hepatitis. In the 1960s, Deinhardt inoculated tamarin monkeys with this serum and at least one of the animals appeared to have contracted hepatitis from the inoculation (8). Nearly three decades later, two agents, GBV-A and GBV-B, were cloned from tamarin serum representing a serial passage of the original tamarin serum (25). A related virus, GBV-C (24) or hepatitis G virus (14), was cloned from human serum. All three viruses are closely related to hepatitis C virus (HCV), with GBV-B being the only hepatotropic virus and the most closely related to HCV (18, 20). At the time of the original GBV studies, it was assumed that the hepatitis agent originated from the serum of the surgeon; however, in retrospect, it seems probable that the agent was already present in the tamarin and that GBV-B is a tamarin virus. This assumption is based on the fact that GBV-B has not been recovered from humans and the fact that GBV-B has a very narrow host range for tamarins and other closely related New World monkeys (R. E. Lanford, unpublished data). The fact that it has not been recovered a second time from tamarins may be due to the rapid resolution of the acute infection in tamarins and the limited number of wild caught tamarins that have been examined immediately upon introduction into captivity.
We have initiated studies with GBV-B, because it represents a surrogate model for HCV. There are three major limitations to working with HCV that can be overcome using the GBV-B system. First, HCV replicates at such low levels that it can be detected only by reverse transcription-PCR (RT-PCR), and viral antigens are difficult to detect reproducibly. Second, the only animal model is the chimpanzee, and chimpanzees are quite large and expensive to use in antiviral studies. Third, no satisfactory tissue culture system has been developed for HCV. In contrast to HCV, GBV-B replicates at levels 1,000- to 10,000-fold higher than those for HCV, the tamarin is about 100 times smaller than the chimpanzee, and we have developed a robust tissue culture system for GBV-B using primary tamarin hepatocytes (1). Although the recently developed (16) and improved (2, 15) HCV replicon system will greatly advance many types of studies with HCV, it cannot replace the need for a virus-based culture system and a small-animal model.
HCV and GBV-B polyproteins possess approximately 25 to 30% homology at the amino acid level (18), while the 5′ and 3′ untranslated regions are more distinct (4, 18, 21). This high level of homology has led to the anticipation that antiviral compounds developed for HCV will be active against GBV-B. This concept is supported by the observation that the GBV-B NS3 protease correctly processes the HCV polyprotein (22) and that HCV–GBV-B chimeric NS3 proteins are enzymatically active (5). In addition, an infectious cDNA clone of GBV-B that induced hepatitis upon intrahepatic inoculation of tamarins with in vitro-transcribed RNA has been produced (4). These studies will certainly be extended to determine whether viable chimeric viruses between HCV and GBV-B can be produced.
In this study, we extend the utility of the tamarin primary hepatocyte culture system for GBV-B to the analysis of antiviral compounds. Interferon (IFN) and ribavirin were evaluated for the ability to reduce GBV-B RNA levels in culture. Both human IFNα-2b and poly(I-C) [polyinosinic acid-poly(C)] resulted in reduction of GBV-B RNA levels. Ribavirin treatment resulted in a dramatic decline in viral RNA levels, which appeared to result from the incorporation of ribavirin triphosphate and the induction of error-prone replication. The results of these studies validate the GBV-B–tamarin hepatocyte system for antiviral testing and suggest that nucleoside analogues that are efficiently incorporated and induce error-prone replication may be efficacious for the treatment of HCV.
(This work was presented in part at the American Society for Virology Annual Meeting in July 2000.)
MATERIALS AND METHODS
Animals.
Moustached tamarins (Saguinus mystax) were housed at the Southwest Regional Primate Research Center at the Southwest Foundation for Biomedical Research. Animals were cared for by members of the Department of Laboratory Animal Medicine in accordance with the Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the Institutional Animal Care and Use Committee. Ribavirin therapy was accomplished by feeding tamarins marshmallows containing the antiviral compound.
Hepatocyte cultures.
Primary tamarin hepatocytes were isolated by collagenase perfusion as previously described (1, 13). Cells were frozen in liquid nitrogen at the time of isolation and were revived and plated on collagen-coated culture dishes as needed. Cultures were maintained in a hormonally defined, serum-free medium (13). Cells were grown in six-well dishes for 3 days prior to infection. Inoculations were performed with 5 μl of GBV-B containing tamarin serum (2 × 105 to 4 × 106 genome equivalents) in 1 ml of serum-free medium for 6 h at 37°C followed by two washes to remove residual inoculum. Poly(I-C) was obtained from Sigma. Ribavirin (1-β-d-ribofuranosyl-1H-1,2,4-triazole-3-carboximide) and human IFNα-2b (Intron A) were obtained from Schering-Plough Research Institute (Kenilworth, N.J.).
TaqMan quantification of GBV-B RNA.
GBV-B RNA was isolated from cells or medium by extraction with RNazol (Leedo, Houston, Tex.), and total cell RNA was quantified by optical density. GBV-B RNA was quantified by a real-time, 5′ exonuclease RT-PCR (TaqMan) assay using a primer-probe combination that recognized a portion of the GBV-B capsid gene as previously described (1). The primers (558F, 5′AACGAGCAAAGCGCAAAGTC; 626R, 5′CATCATGGATACCAGCAATTTTGT) and probe (579P; 5′6FAM-AGCGCGATGCTCGGCCTCGTA-TAMRA) were selected using the Primer Express software designed for this purpose (PE Applied Biosystems, Foster City, Calif.) and were purchased from PE Applied Biosystems. Standards to establish genome equivalents were synthetic RNAs transcribed from the cloned GBV-B capsid gene.
Anti-NS3 ELISA.
Antibodies to NS3 in GBV-B-infected animals were monitored with an enzyme-linked immunoadsorbent assay (ELISA) using purified NS3 (1). Purified NS3 protein (10 ng per well) was bound to 96-well Immunlon 2 plates (Dynatech Laboratories, Chantilly, Va.) in borate-buffered saline (145 mM NaCl, 6 mM NaOH, 48 mM H3BO3, and 50 mM KCl to give a pH of 8.2) overnight at 4°C. All ELISA incubations were performed for 1 h at 37°C, except for the final substrate incubation, and between incubation steps, wells were washed four times with phosphate-buffered saline (PBS)–0.05% Tween 20. Unoccupied protein binding sites were blocked with 5% bovine serum albumin (BSA) in PBS. Serial tamarin serum samples were diluted 1:40 in antibody diluent, 0.5% BSA–PBS–0.05% Tween 20. Bound antibody was detected with goat anti-human immunoglobulin G–horseradish peroxidase conjugate diluted 1:1,000 in antibody diluent. The substrate {100 μl of 1 mg/ml ABTS [2,2′-azinobis(3-ethylbenthiazolinesulfonic acid]) [Sigma] in 0.03% H2O2} was incubated at room temperature until color development was stopped by the addition of 50 μl of 1% sodium dodecyl sulfate. Plates were read at 405 nm.
apoB ELISA.
The ELISA for apolipoprotein B (apoB) was performed with culture medium from primary cultures of tamarin hepatocytes as previously described (12). Microtiter wells were coated with 100 μl of anti-apoB polyclonal antibody (Biodesign, Kennebunkport, Maine) per well at 1.5 μg/ml in 0.1 M sodium bicarbonate buffer, pH 9.0. Wells were blocked with PBS containing 0.05% Tween 20 and 3% BSA. Wells were washed one time with PBS–0.05% Tween 20 and were incubated for 3 h at 37°C with 100 μl of culture medium. Wells were washed three times with PBS–0.05% Tween 20 and incubated for 2 h at 37°C with alkaline phosphatase-conjugated antibody diluted in PBS–0.05% Tween 20 containing 1% BSA. Anti-apoB horseradish peroxidase-conjugated antibody was obtained from The Binding Site (San Diego, Calif). Wells were washed three times with PBS–0.05% Tween 20 and incubated for 15 min with citrate buffer, pH 5.0, containing 0.3% H2O2 and 5 μg of o-phenylenediamine dihydrochloride per ml. The reaction was stopped with 4 N H2SO4, and absorbance at 490 nm was determined. The estimates of sample concentrations (in nanograms per milliliter) were calculated using a regression equation fitted to a standard curve based on log-log transformed optical density data (12).
RESULTS
Inhibition of GBV-B replication by IFN.
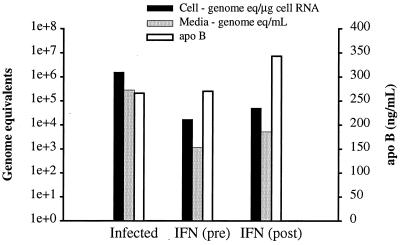
We have previously described a tissue culture system for in vitro replication of GBV-B in primary tamarin hepatocyte cultures. We were interested in using this system to evaluate antiviral compounds. Since no antivirals have been developed for GBV-B, we chose to examine the two drugs used for the treatment of HCV infections, IFN and ribavirin. The initial studies involved the treatment of cultures with human IFNα-2b at high levels (Intron A; 2,000 U/ml) because of the species difference between the sources of the IFN and hepatocytes. In addition, our studies with HCV replicons in Huh7 cells have suggested that an additional antiviral effect is evident with concentrations as high as 1,000 U/ml (Lanford, unpublished). IFN treatment was started either the day before inoculation or 1 day after inoculation. The in vitro growth curve of GBV-B in primary tamarin hepatocytes demonstrated that maximum intracellular viral RNA levels were reached by 1 day postinfection (1). Thus, the treatment of cultures at 1 day postinfection reflects treatment of an established infection, and any decline in the levels of viral RNA should be the result of loss of viral RNA following inhibition of replication. In contrast, treatment of cultures prior to infection requires only the inhibition of replication, with no requirement for the loss of existing RNA as detected by RT-PCR. Treatment with IFN was continued for 7 days following infection. Regardless of whether treatment was initiated before or after infection, the levels of viral RNA in the cells and medium were suppressed by approximately 2 log units as determined by quantitative, real-time (TaqMan) RT-PCR (Fig. 1). To control for any nonspecific, adverse effects of the treatment on the hepatocyte cultures, the synthesis and secretion of apoB was monitored in all cultures using a quantitative ELISA. This liver-specific function was monitored, because it represents a very sensitive indicator of hepatocyte differentiated function. No significant decrease in apoB was observed. Additional experiments indicated that lower doses of IFN had little to no antiviral effect and that the level of viral suppression obtained with human IFN was both marginal and variable. The limited effect of human IFN on GBV-B replication was potentially due to the species differences between the IFN source and the target tissue source (tamarins), since studies with HCV replicons have demonstrated a pronounced inhibition of HCV replication with IFN (2, 15) (Lanford, unpublished).
FIG. 1.
Antiviral effect of IFN on GBV-B-infected tamarin hepatocytes. Primary tamarin hepatocytes were treated with human IFN (IFNα-2b; 2,000 U/ml) starting the day before or the day after infection with GBV-B. IFN treatment was continued for 7 days with medium changes and fresh IFN every other day. The levels of viral RNA in the cells (in genome equivalents per microgram of cell RNA) and medium (in genome equivalents per milliliter of medium) were determined by real-time TaqMan RT-PCR. IFN treatment resulted in an approximately 2-log reduction of cell-associated and secreted viral RNA. No inhibition of cellular functions was noted, as indicated by the secretion of apoB, a highly differentiated marker for hepatocytes. On the left y axis, genome equivalents are shown on a logarithmic scale (1e+0, 100; 1e+1, 101; 1e+2, 102, etc.).
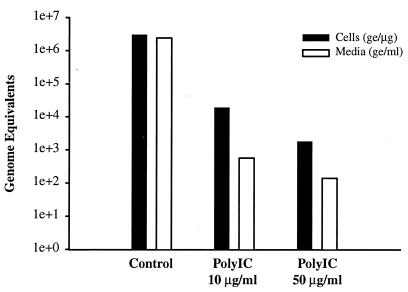
Next, cultures were treated with poly(I-C) which not only induces the synthesis of endogenous IFN but also activates the IFN-inducible, antiviral pathways of protein kinase R and 2′,5′-oligoadenylate synthetase and thus, RNase L. Cultures were treated in duplicate with 10 and 50 μg of poly(I-C) per ml. Treatment was initiated the day prior to infection and was continued until the time of harvest, 7 days postinfection. Analysis of cell-associated viral RNA from treated and untreated cultures revealed 2.2-log and 3.3-log decreases in viral RNA levels with 10 and 50 μg of poly(I-C) per ml, respectively (Fig. 2). The decline in secreted viral RNA levels was greater with 3.6-log and 4.2-log decreases at 10 and 50 μg of poly(I-C) per ml. The 3.3-log decrease in cell-associated viral RNA at 50 μg/ml may reflect an experimental limitation, since the residual cell-associated viral RNA that remains after antiviral treatment represents less than 0.1% of the input inoculum. This level of RNA may represent the amount of inoculum that adheres to the plastic wells and cultures in a nonproductive, yet stable manner. The RNA present in viral particles adsorbed on the plastic surface can persist for an extended period of time as detected by RT-PCR. Thus, in this scenario, even 100% inhibition of viral replication would not exceed 3.3 log units of reduction of cell-associated viral RNA in comparison to untreated cultures. When these data are expressed in another manner, treatment with 50 μg of poly(I-C) per ml yielded >99.9% reduction of cell-associated viral RNA and >99.99% reduction in the level of viral RNA secreted into the culture medium.
FIG. 2.
Antiviral effect of poly(I-C) on GBV-B-infected tamarin hepatocytes. Primary tamarin hepatocytes were treated with 10 or 50 μg of poly(I-C) per ml beginning the day before infection with GBV-B. Treatment was continued for 7 days with medium changes and fresh poly(I-C) every other day. The levels of viral RNA in the cells (in genome equivalents [ge] per microgram of cell RNA) and medium (in genome equivalents per milliliter of medium) were determined by real-time TaqMan RT-PCR. Poly(I-C) treatment resulted in greater than 3 and 4 log units of reduction of cell-associated and secreted viral RNA, respectively. On the left y axis, genome equivalents are shown on a logarithmic scale (1e+0, 100; 1e+1, 101; 1e+2, 102, etc.).
To determine whether poly(I-C) eliminated all infectious viral RNA, cultures were treated with poly(I-C) for 7 or 14 days, and some of the cultures treated for 14 days were grown in the absence of poly(I-C) for an additional 7 days. No further decline in viral RNA levels occurred between days 7 and 14 of treatment, and no increase in viral RNA occurred following removal of poly(I-C) for 7 days (data not shown). These data suggest that no infectious viral RNA remained in the cells or that an antiviral state persisted in the cells after the removal of poly(I-C). In a separate experiment, infected cultures were treated with poly(I-C) for 7 days. The medium was used to inoculate fresh hepatocytes, and this process was repeated after another 7 days. GBV-B RNA levels in the cells decreased only 0.65 log unit during serial passage of virus from untreated cultures (Table 1), while GBV-B RNA declined to below the levels of detection in cells and media during passage of the poly(I-C)-treated culture medium. Although this experiment was initially designed to select and expand IFN-resistant variants, it demonstrates the near total inhibition of viral replication by poly(I-C). No IFN-resistant variants were selected using this protocol.
TABLE 1.
Effect of poly(I-C) on viral RNA levels for tamarin hepatocytes infected with GBV-B
| Poly(I-C)a | Sample | Viral RNA level (log10 ge) at passage no.b:
|
||
|---|---|---|---|---|
| 0 | 1 | 2 | ||
| + | Cells | 3.43 | 1.11 | 0 |
| Medium | 3.18 | 0 | 0 | |
| − | Cells | 5.65 | 5.11 | 5.00 |
| Medium | 5.61 | 4.80 | 4.62 | |
Tamarin hepatocytes were cultured in the presence (+) or absence (−) of 50 μg of poly(I-C) per ml starting 1 day before infection with GBV-B and for 7 days after infection.
Cultures were harvested for determination of viral RNA levels (log10 ge, log10 value of genome equivalent per microgram of cell RNA or genome equivalents per milliliter of medium). The culture medium from day 7 was used to inoculate fresh cultures (passage 1). The process was repeated a second time (passage 2).
Treatment of GBV-B-infected cultures with ribavirin.
Next, ribavirin was examined for an antiviral effect on GBV-B-infected tamarin hepatocytes. The monophosphate form of ribavirin is an IMP dehydrogenase (IMPDH) inhibitor that has an antiviral effect for a number of viruses (7, 17, 23). The antiviral effect is due in part to the reduction of GTP pools by inhibition of IMPDH. Although ribavirin in combination with IFN is used for the treatment of HCV infections, ribavirin monotherapy induces an improvement in liver disease without a reduction in the level of viremia (3, 9). The mechanism is believed to involve an immunomodulatory activity possessed by ribavirin that promotes a Th1-biased immune response (10, 11, 19, 26).
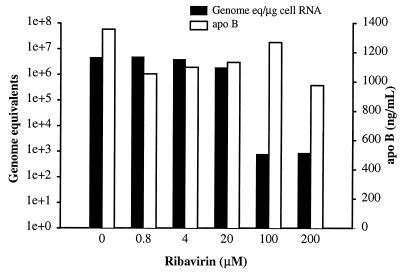
Surprisingly, treatment of GBV-B-infected tamarin hepatocytes with ribavirin resulted in a dramatic decline in viral RNA levels (Fig. 3). Ribavirin treatment was initiated at various concentrations the day before infection, and cultures were harvested 6 days after infection. At the two highest levels of ribavirin employed (100 and 200 μM), a 3.8-log decrease in cell-associated viral RNA levels was observed. No overt toxicity was observed in any of the cultures by microscopic examination; however, at 200 μM, a small decrease in apoB secretion was observed. Thus, ribavirin appears to function as a true antiviral in the GBV-B–tamarin hepatocyte system. The level of ribavirin at which antiviral activity was observed (100 μM) is probably higher than that obtained for patients on ribavirin therapy.
FIG. 3.
Suppression of GBV-B replication by ribavirin. Primary tamarin hepatocytes were treated with various concentrations of ribavirin (0 to 200 μM) starting the day before infection. Treatment was continued for 7 days with fresh medium, with ribavirin provided every other day. The level of viral RNA in the cells (in genome equivalents per microgram of cell RNA) was determined by real-time TaqMan RT-PCR. No inhibition of the secretion of apoB, a marker of hepatocyte function, was noted except possibly at the highest ribavirin concentration. At 100 μM ribavirin, an approximately 4-log reduction in cell-associated viral RNA was observed. On the left y axis, genome equivalents are shown on a logarithmic scale (1e+0, 100; 1e+1, 101; 1e+2, 102, etc.).
Serial passage of medium from ribavirin-treated cultures was performed in a manner similar to that described above for poly(I-C). Cultures were treated with 100 μM ribavirin beginning the day prior to infection, and treatment was maintained for 7 days. The medium from treated and untreated cultures was passed two additional times to fresh cultures. No virus was detected in the cells or the medium after the first passage of the treated medium, while the medium for untreated cultures efficiently initiated new infections (Table 2). These data imply that ribavirin treatment resulted in near complete inhibition of viral replication and/or secretion of infectious virus and that using the current experimental design no ribavirin-resistant mutants emerged.
TABLE 2.
Effect of ribavirin on viral RNA levels for tamarin hepatocytes infected with GBV-B
| Ribavirina | Sample | Viral RNA level (log10 ge) at passage no.b:
|
||
|---|---|---|---|---|
| 0 | 1 | 2 | ||
| + | Cells | 3.99 | 0 | 0 |
| Medium | 2.86 | 0 | 0 | |
| − | Cells | 5.92 | 5.28 | 5.15 |
| Medium | 5.85 | 5.11 | 4.53 | |
Tamarin hepatocytes were cultured in the presence (+) or absence (−) of 100 μM ribavirin starting 1 day before infection with GBV-B and for 7 days after infection.
Cultures were harvested for determination of viral RNA levels (log10 ge, log10 value of genome equivalents per microgram of cell RNA or genome equivalents per milliliter of medium). The culture medium from day 7 was used to inoculate fresh cultures (passage 1). The process was repeated a second time (passage 2).
Guanosine abolishes the antiviral effect of ribavirin.
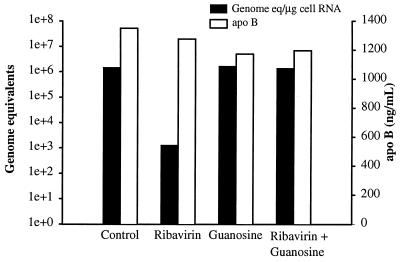
We were interested in the mechanism of the antiviral effect of ribavirin. Ribavirin could affect viral RNA synthesis by suppressing intracellular pools of GTP. However, apoB synthesis was not affected even over a 7-day treatment (Fig. 3), and the apoB mRNA is larger than the GBV-B genome. Thus, it was questionable whether inhibition of RNA synthesis by reduction of GTP pools was the primary mechanism of the antiviral effect observed in vitro. The role of intracellular GTP pools was examined by competing ribavirin with excess guanosine in the culture medium, which would provide GTP through an alternate metabolic pathway. No effect on GBV-B replication was observed with guanosine supplementation alone, while guanosine supplementation at 100 μM completely eliminated the antiviral effect of ribavirin (Fig. 4). These data suggest that a reduction in GTP pools was required for the ribavirin effect, but there was still the possibility that the primary antiviral effect was not due solely to inhibition of RNA synthesis at low GTP levels, especially in the absence of overt cellular toxicity or inhibition of apoB synthesis.
FIG. 4.
Guanosine supplementation abolishes the ribavirin antiviral effect on GBV-B replication. Primary tamarin hepatocytes were treated with ribavirin (100 μM) with or without guanosine supplementation (100 μM). Treatment was initiated the day before infection and continued for 7 days with fresh medium, with ribavirin and guanosine provided every other day. The level of viral RNA in the cells (in genome equivalents per microgram of cell RNA) was determined by real-time TaqMan RT-PCR. Guanosine supplementation completely abolished the antiviral effect of ribavirin, but guanosine alone had no observable effect on GBV-B replication. On the left y axis, genome equivalents are shown on a logarithmic scale (1e+0, 100; 1e+1, 101; 1e+2, 102, etc.).
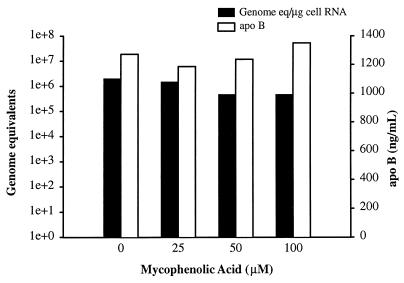
Lack of antiviral effect of MPA.
To further examine the role of GTP pools in the antiviral effect of ribavirin, a different inhibitor of IMPDH was examined. While ribavirin is a guanosine analogue and is a competitive inhibitor of IMPDH, mycophenolic acid (MPA) is an uncompetitive inhibitor of IMPDH. Tamarin hepatocytes were treated with MPA and infected with GBV-B using a protocol identical to that used for ribavirin with pretreatment for 1 day prior to infection and continued treatment for 7 days postinfection. No effect on GBV-B replication was observed at 100 μM, the highest concentration of MPA employed (Fig. 5). Although no direct demonstration of IMPDH inhibition by MPA was performed in these studies, the inhibition of IMPDH is a well-established activity of MPA. Concentrations above 100 μM were cytotoxic. These data imply that although required for the antiviral effect, reduction of GTP pools was probably not the primary antiviral mechanism of ribavirin.
FIG. 5.
MPA does not significantly inhibit GBV-B replication. Primary tamarin hepatocytes were treated with various concentrations of MPA (0 to 100 μM) starting the day before infection. Treatment was continued for 7 days with fresh medium, with MPA provided every other day. The level of viral RNA in the cells (in genome equivalents per microgram of cell RNA) was determined by real-time TaqMan RT-PCR. No inhibition of GBV-B replication was noted even at the highest nontoxic dose of MPA. On the left y axis, genome equivalents are shown on a logarithmic scale (1e+0, 100; 1e+1, 101; 1e+2, 102, etc.).
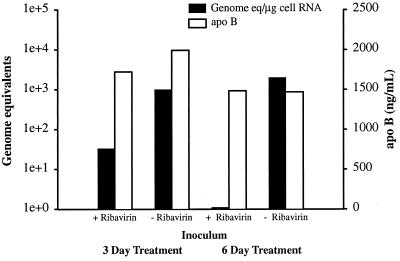
Reduction of specific infectivity of GBV-B by ribavirin treatment.
One possibility consistent with the contrasting effects of different IMPDH inhibitors and the elimination of the ribavirin effect by guanosine supplementation was the incorporation of ribavirin triphosphate (RTP) by the GBV-B polymerase. Sufficient incorporation of RTP would occur only when the GTP pool was suppressed, because the GBV-B polymerase would favor utilization of GTP over RTP. This hypothesis could be tested by examining the effect of ribavirin on the specific infectivity of GBV-B produced in the presence of ribavirin. If RTP were incorporated into GBV-B RNA, in the next round of RNA synthesis, RMP would be copied by the GBV-B polymerase as either a guanosine or adenosine, which would lead to error-prone replication. The accumulation of errors would in turn decrease the infectivity of the virus.
To test this hypothesis, hepatocytes were infected with GBV-B in the presence or absence of ribavirin, the secreted virions were harvested, and the harvested virions were adjusted such that the treated and untreated virus had the same number of genomic equivalents by RT-PCR. These media were then used to infect fresh cultures of hepatocytes. Virus grown in the presence of ribavirin for 3 days had a markedly reduced infectivity (Fig. 6), while virus grown in the presence of ribavirin for 6 days was essentially noninfectious. Repetition of the experiment produced essentially identical results. These data are highly suggestive of induction of error-prone replication due to incorporation of RTP.
FIG. 6.
GBV-B produced in the presence of ribavirin has low specific infectivity. Primary tamarin hepatocytes were cultivated with (+) or without (−) 100 μM ribavirin as described in the legend to Fig. 3. Secreted virus was harvested on day 3 or 6 postinfection with GBV-B. The virus levels in the media were adjusted to contain identical genome equivalents based on TaqMan RT-PCR. The media were then used to inoculate fresh cultures of hepatocytes. The infected cultures were harvested 7 days postinoculation, and the level of cell-associated viral RNA (in genome equivalents per microgram of cell RNA) was determined as a measure of specific infectivity of the inoculum. Infectivity of GBV-B harvested after 3 days of ribavirin treatment was decreased 2 log units, while GBV-B harvested after 6 days of ribavirin treatment lacked measurable infectivity. The small bar present for the sample treated with ribavirin for 6 days shows that the sample was tested but no viral RNA was detected. On the left y axis, genome equivalents are shown on a logarithmic scale (1e+0, 100; 1e+1, 101; 1e+2, 102, etc.).
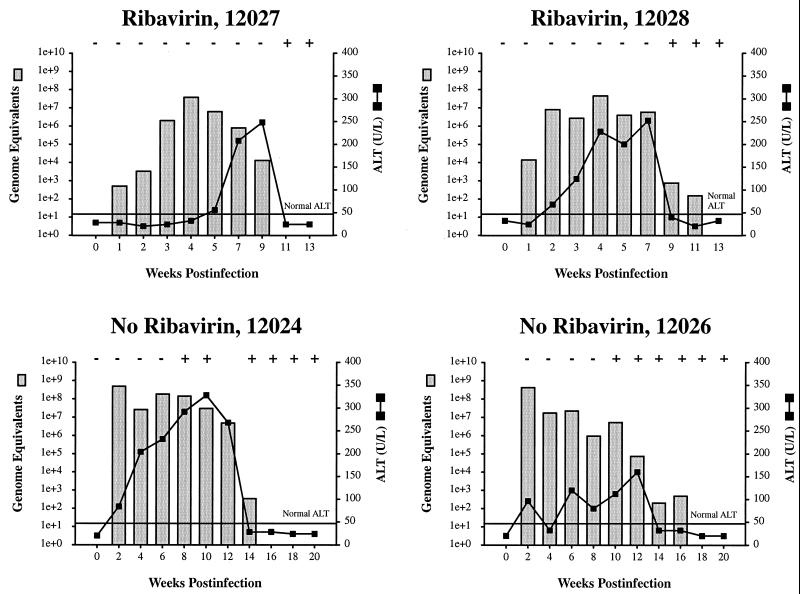
Lack of antiviral activity of ribavirin in GBV-B-infected tamarins.
The high efficacy of ribavirin against GBV-B replication in vitro prompted an extension of the studies to include the treatment of GBV-B-infected tamarins. Tamarins were fed ribavirin at 10 mg per day (approximately 20 mg/kg of body weight), a dose approximately 30% higher than that used for HCV-infected patients. Animals were treated with ribavirin for 7 days prior to GBV-B inoculation, such that steady-state RTP levels would be present in the liver at the time of infection, and treatment was continued for 10 days postinoculation. The early bleed schedules used for the animals not treated with ribavirin differed from the animals treated with ribavirin, such that the graphs cannot be directly compared; however, ribavirin monotherapy did not prevent establishment of the infection, did not result in a reduced peak of viremia, and did not result in rapid clearance of GBV-B infection (Fig. 7). Although the initial viremia level was lower in one of the two ribavirin-treated animals (compare week 2 in treated and untreated animals), no major impact on replication was observed. The untreated animals represent profiles from a previous experiment (1) in which week 1 samples were not available for comparison. There was significant variation observed between individual animals with regard to the early levels of viremia, so the reduced viremia prior to week 4 in ribavirin-treated animals is probably not significant. A gradual increase in the levels of viremia prior to week 4 has been observed in other GBV-B-infected animals. These data imply that GBV-B is not particularly sensitive to ribavirin at the concentrations typically used in vivo and in this respect resembles HCV. Much higher, potentially toxic levels of ribavirin would be required to obtain significant antiviral efficacy in vivo.
FIG. 7.
Ribavirin therapy does not alter the course of GBV-B infection in tamarins. Tamarins were fed ribavirin at 10 mg per day (approximately 20 mg/kg) starting 7 days prior to inoculation with GBV-B, and treatment was continued for the first 10 days postinoculation. The level of viremia at various times after inoculation was determined by real-time TaqMan RT-PCR and is indicated by stippled bars. Serum alanine transaminase (ALT) levels were monitored as a biochemical indication of liver damage and are indicated by solid lines. The horizontal line indicates the upper normal limit for ALT. Seroconversion for GBV-B anti-NS3 was monitored by ELISA (indicated by symbols above the graph as follows: −, seroconversion did not occur; +, seroconversion occurred). The animals 12027 and 12028 (top) were treated with ribavirin, while animals 12024 and 12026 (bottom) were not treated. The profiles for animals 12024 and 12026 were previously published (1). The bleed schedules used for the animals not treated with ribavirin differed from the animals treated with ribavirin, so the graphs cannot be directly compared; however, ribavirin monotherapy did not appear to alter the course of GBV-B infection. On the left y axis, genome equivalents are shown on a logarithmic scale (1e+0, 100; 1e+1, 101; 1e+2, 102, etc.).
DISCUSSION
The GBV-B–tamarin system provides a powerful surrogate system for HCV. The high level of replication facilitates detection of viral replication and viral antigens (1), and the tamarin provides a useful small-animal model for in vivo studies. The development of an efficient culture system for GBV-B permits a number of studies not easily performed for HCV, despite the fact that the system is dependent upon the use of primary hepatocyte cultures. In this report, we demonstrate the utility of the culture system and animal model for antiviral studies. As anticipated, GBV-B replication was inhibited by IFN. Human IFN had a reduced antiviral effect in comparison to poly(I-C). Poly(I-C) has the capacity to induce endogenous tamarin IFN as well as protein kinase R and 2′,5′OAS antiviral pathways. The reduced antiviral effect of human IFN was presumably due to a reduced affinity for the type I IFN receptor on tamarin hepatocytes, although direct demonstration of this will require additional studies. Ribavirin was tested, since it is currently being used in the clinic for HCV infection. However, the clinical data suggested that it would probably not have significant antiviral activity against GBV-B. The finding that ribavirin is a highly efficacious antiviral compound for GBV-B demonstrates the value of this in vitro system for the analysis of a broad variety of antiviral compounds.
Ribavirin has an antiviral effect on a number of viruses including both RNA and DNA viruses (7, 17, 23). Presumably, the antiviral effect in most cases is mediated by inhibition of IMPDH and reduction of intracellular pools of GTP and dGTP. Such a mechanism would account for the broad array of viruses susceptible to inhibition by ribavirin but would at the same time raise questions with regard to the lack of sensitivity of some viruses to ribavirin inhibition. However, different viruses are often assayed in different cell types under a variety of culture conditions. Of importance may be the level of ribavirin monophosphate available for inhibition of IMPDH, the utilization of salvage pathways to produce GTP, and the demand on GTP pools due to cellular growth. Ribavirin can potentiate the antiviral activity of other guanosine-based nucleoside analogues for hepatitis B virus, presumably because the analogues are more efficiently utilized once dGTP levels are reduced by ribavirin (27). The requirement for reduced GTP levels for the ribavirin-induced inhibition of GBV-B was apparent by the reversal of the antiviral effect by guanosine supplementation. An alternative but similar explanation is that ribavirin does not significantly reduce GTP pools in primary tamarin hepatocytes under the conditions employed and that error-prone replication occurs in the presence of normal GTP levels in this system, but excessive GTP levels due to guanosine supplementation ablate the effect. In some studies, the antiviral effect of ribavirin is not abolished by guanosine supplementation (17), suggesting that ribavirin may possess other mechanisms of antiviral activity. The potent antiviral profile of another IMPDH inhibitor (VX-497) resembles but is not identical to that of ribavirin, suggesting that for most viruses the IMPDH inhibition provides the antiviral effect independent of the guanosine-like structure of ribavirin (17). The studies in this report are among the first to suggest that the antiviral effect of ribavirin for some viruses is exerted by incorporation of RTP and induction of error-prone replication.
During preparation of this report, studies with poliovirus that also concluded that ribavirin can act as a mutagen due to error-prone replication were published (6). In these studies, in vitro assays with the poliovirus polymerase demonstrated incorporation of RTP into a synthetic template, and treatment of poliovirus-infected cells with ribavirin resulted in error-prone replication, as measured by an increase in the frequency of guanosine-resistant mutants and by sequencing of the poliovirus genome. The level of ribavirin required for a significant antiviral effect was much higher for poliovirus (1,000 μM) than that required in our studies on GBV-B (100 μM). There are several possible explanations for this discrepancy: the cellular uptake of ribavirin and conversion to RTP may differ between the cell types used in the poliovirus studies and the primary tamarin hepatocytes; the fact that primary hepatocytes are nondividing cultures may have a significant impact on intracellular GTP pools; the degree to which the salvage pathways are used to supply GTP will also influence the efficacy of ribavirin, as demonstrated in the guanosine supplementation studies (Fig. 4); and the relative affinity of the two viral polymerases for RTP may differ as well. The disassociation constants for the poliovirus polymerase were measured in an in vitro assay using a synthetic template in which the addition of a templated GMP could be measured. The Kd for ribavirin was 113-fold higher than the Kd for GTP, 430 μM versus 3.8 μM, respectively. The disassociation constant for the GBV-B polymerase cannot be measured at this time due to the lack of an in vitro assay for the purified polymerase.
The immediate assumption from the poliovirus and GBV-B studies is that nucleoside analogues that induce error-prone replication should be highly efficacious in the treatment of viral infections, especially RNA viruses, and importantly HCV infections. However, one must consider the specific circumstances in which ribavirin exerts its effect. Ribavirin must first reduce the levels of the natural nucleotide triphosphate with which it must compete for incorporation. With ribavirin, this is facilitated by virtue of its inhibition of an essential enzyme upstream in the pathway for nucleotide triphosphate synthesis. The efficacy of other guanosine analogues could be potentiated using combination therapy with ribavirin, as has been observed for hepatitis B virus (27). For the use of analogues for other nucleosides to induce error-prone replication, either the biosynthetic pathway for the production of the natural triphosphate must be inhibited or the viral enzyme must have a very low Kd for the nucleotide triphosphate form of the analogue. Nonetheless, the potential for induction of error-prone replication as an antiviral strategy is particularly attractive for RNA viruses.
ACKNOWLEDGMENTS
This work was supported in part by grants RO1 AI49574 and P51 RR13986 from the National Institutes of Health and by a grant from the Schering-Plough Research Institute.
REFERENCES
- 1.Beames B, Chavez D, Guerra B, Notvall L, Brasky K M, Lanford R E. Development of a primary tamarin hepatocyte culture system for GB virus-B: a surrogate model for hepatitis C virus. J Virol. 2000;74:11764–11772. doi: 10.1128/jvi.74.24.11764-11772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blight K J, Kolykhalov A A, Rice C M. Efficient initiation of HCV RNA replication in cell culture. Science. 2000;290:1972–1974. doi: 10.1126/science.290.5498.1972. [DOI] [PubMed] [Google Scholar]
- 3.Bodenheimer H C, Jr, Lindsay K L, Davis G L, Lewis J H, Thung S N, Seeff L B. Tolerance and efficacy of oral ribavirin treatment of chronic hepatitis C: a multicenter trial. Hepatology. 1997;26:473–477. doi: 10.1002/hep.510260231. [DOI] [PubMed] [Google Scholar]
- 4.Bukh J, Apgar C L, Yanagi M. Toward a surrogate model for hepatitis C virus: an infectious molecular clone of the GB virus-B hepatitis agent. Virology. 1999;262:470–478. doi: 10.1006/viro.1999.9941. [DOI] [PubMed] [Google Scholar]
- 5.Butkiewicz N, Yao N, Zhong W, Wright-Minogue J, Ingravallo P, Zhang R, Durkin J, Strandring D N, Baroudy B M, Sangar D V, Lemon S M, Lau J Y N, Hong Z. Virus-specific cofactor requirement and chimeric hepatitis C virus/GB virus B nonstructural protein 3. J Virol. 2000;74:4291–4301. doi: 10.1128/jvi.74.9.4291-4301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crotty S, Maag D, Arnold J J, Zhong W, Lau J N, Hong Z, Andino R, Cameron C E. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat Med. 2000;6:1375–1379. doi: 10.1038/82191. [DOI] [PubMed] [Google Scholar]
- 7.De Clercq E. Antiviral agents: characteristic activity spectrum depending on the molecular target with which they interact. Adv Virus Res. 1993;42:1–55. doi: 10.1016/s0065-3527(08)60082-2. [DOI] [PubMed] [Google Scholar]
- 8.Deinhardt F, Holmes A W, Capps R B, Popper H. Studies on the transmission of human viral hepatitis to marmoset monkeys. J Exp Med. 1967;125:673–688. doi: 10.1084/jem.125.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dusheiko G, Main J, Thomas H, Reichard O, Lee C, Dhillon A, Rassam S, Fryden A, Reesink H, Bassendine M, Norkrans G, Cuypers T, Lelie N, Telfer P, Watson J, Weegink C, Sillikens P, Weiland O. Ribavirin treatment for patients with chronic hepatitis C: results of a placebo-controlled study. J Hepatol. 1996;25:591–598. doi: 10.1016/s0168-8278(96)80225-x. [DOI] [PubMed] [Google Scholar]
- 10.Fang S H, Hwang L H, Chen D S, Chiang B L. Ribavirin enhancement of hepatitis C virus core antigen-specific type 1 T helper cell response correlates with the increased IL-12 level. J Hepatol. 2000;33:791–798. doi: 10.1016/s0168-8278(00)80312-8. [DOI] [PubMed] [Google Scholar]
- 11.Hultgren C, Milich D R, Weiland O, Sällberg M. The antiviral compound ribavirin modulates the T helper (Th)1/Th2 subset balance in hepatitis B and C virus-specific immune responses. J Gen Virol. 1998;79:2381–2391. doi: 10.1099/0022-1317-79-10-2381. [DOI] [PubMed] [Google Scholar]
- 12.Lanford R E, Estlack L, White A L. Neomycin inhibits secretion of apolipoprotein[a] by increasing retention on the hepatocyte cell surface. J Lipid Res. 1996;37:2055–2064. [PubMed] [Google Scholar]
- 13.Lanford R E, Estlack L E. A cultivation method for highly differentiated primary chimpanzee hepatocytes permissive for hepatitis C virus replication. Methods Mol Med. 1998;19:501–516. doi: 10.1385/0-89603-521-2:501. [DOI] [PubMed] [Google Scholar]
- 14.Linnen J, Wages J, Jr, Zhang-Keck Z-Y, Fry K E, Krawczynski K Z, Alter H, Koonin E, Gallagher M, Alter M, Hadziyannis S, Karayiannis P, Fung K, Nakatsuji Y, Shih J W K, Young L, Piatak M, Jr, Hoover C, Fernandez J, Chen S, Zou J-C, Morris T, Hyams K C, Ismay S, Lifson J D, Hess G, Foung S K H, Thomas H, Bradley D, Margolis H, Kim J P. Molecular cloning and disease association of hepatitis G virus: a transfusion-transmissible agent. Science. 1996;271:505–508. doi: 10.1126/science.271.5248.505. [DOI] [PubMed] [Google Scholar]
- 15.Lohmann V, Korner F, Dobierzewska A, Bartenschlager R. Mutations in hepatitis C virus RNAs conferring cell culture adaptation. J Virol. 2001;75:1437–1449. doi: 10.1128/JVI.75.3.1437-1449.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lohmann V, Körner F, Koch J O, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 17.Markland W, McQuaid T J, Jain J, Kwong A D. Broad-spectrum antiviral activity of the IMP dehydrogenase inhibitor VX-497: a comparison with ribavirin and demonstration of antiviral additivity with alpha interferon. Antimicrob Agents Chemother. 2000;44:859–866. doi: 10.1128/aac.44.4.859-866.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muerhoff A S, Leary T P, Simons J N, Pilot-Matias T J, Dawson G J, Erker J C, Chalmers M L, Schlauder G G, Desai S M, Mushahwar I K. Genomic organization of GB viruses A and B: two new members of the Flaviviridae associated with GB agent hepatitis. J Virol. 1995;69:5621–5630. doi: 10.1128/jvi.69.9.5621-5630.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ning Q, Brown D, Parodo J, Cattral M, Gorczynski R, Cole E, Fung L, Ding J W, Liu M F, Rotstein O, Phillips M J, Levy G. Ribavirin inhibits viral-induced macrophage production of TNF, IL-1, the procoagulant fgl2 prothrombinase and preserves Th1 cytokine production but inhibits Th2 cytokine response. J Immunol. 1998;160:3487–3493. [PubMed] [Google Scholar]
- 20.Ohba K, Mizokami M, Lau J Y N, Orito E, Ikeo K, Gojobori T. Evolutionary relationship of hepatitis C, pesti-, flavi-, plant viruses, and newly discovered GB hepatitis agents. FEBS Lett. 1996;378:232–234. doi: 10.1016/0014-5793(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 21.Rijnbrand R, Abell G, Lemon S M. Mutational analysis of the GB virus B internal ribosome entry site. J Virol. 2000;74:773–783. doi: 10.1128/jvi.74.2.773-783.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scarselli E, Urbani A, Sbardellati A, Tomei L, De Francesco R, Traboni C. GB virus B and hepatitis C virus NS3 serine proteases share substrate specificity. J Virol. 1997;71:4985–4989. doi: 10.1128/jvi.71.7.4985-4989.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sidwell R W, Huffman J H, Khare G P, Allen L B, Witkowski J T, Robins R K. Broad-spectrum antiviral activity of virazole: 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science. 1972;177:705–706. doi: 10.1126/science.177.4050.705. [DOI] [PubMed] [Google Scholar]
- 24.Simons J N, Leary T P, Dawson G J, Pilot-Matias T J, Muerhoff A S, Schlauder G G, Desai S M, Mushahwar I K. Isolation of novel virus like sequences associated with human hepatitis. Nat Med. 1995;1:564–569. doi: 10.1038/nm0695-564. [DOI] [PubMed] [Google Scholar]
- 25.Simons J N, Pilot-Matias T J, Leary T P, Dawson G J, Desai S M, Schlauder G G, Muerhoff A S, Erker J C, Buijk S L, Chalmers M L, Van Sant C L, Mushahwar I K. Identification of two flavivirus-like genomes in the GB hepatitis agent. Proc Natl Acad Sci USA. 1995;92:3401–3405. doi: 10.1073/pnas.92.8.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tam R C, Pai B, Bard J, Lim C, Averett D R, Phan U T, Milovanovic T. Ribavirin polarizes human T cell responses towards a type 1 cytokine profile. J Hepatol. 1999;30:376–382. doi: 10.1016/s0168-8278(99)80093-2. [DOI] [PubMed] [Google Scholar]
- 27.Ying C, De Clercq E, Neyts J. Ribavirin and mycophenolic acid potentiate the activity of guanine- and diaminopurine-based nucleoside analogues against hepatitis B virus. Antiviral Res. 2000;48:117–124. doi: 10.1016/s0166-3542(00)00121-2. [DOI] [PubMed] [Google Scholar]