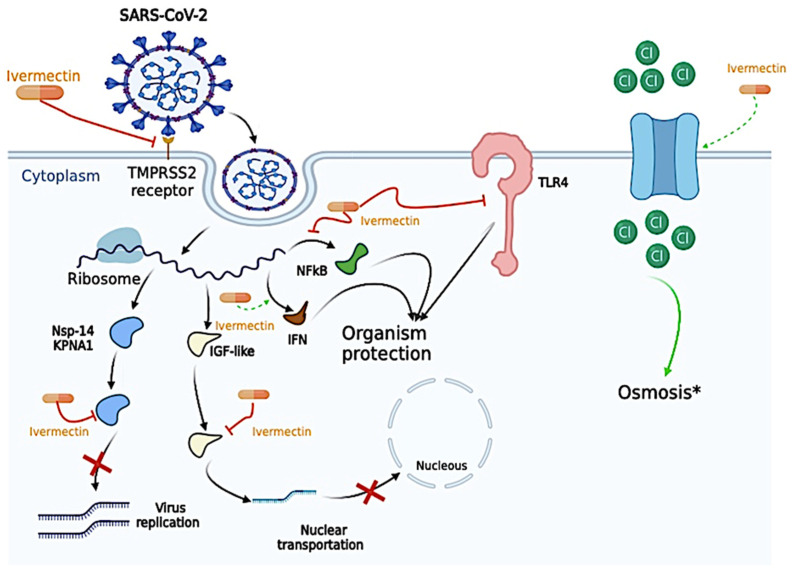
Figure 1.
Proposed antiviral mechanism of Ivermectin. Ivermectin can disrupt the binding of essential proteins that allow cell entrance, such as Transmembrane Serine Protease 2 (TMPRSS2) and the Spike Protein. Ivermectin was also described to (i) bind to the alpha subunit of the insulin-like growth factor (IGF) superfamily and prevent the nuclear transportation of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); (ii) generate apoptosis and osmotic cell death by upregulating chloride channels since Ivermectin molecules behave as ionophores. In the same way, Ivermectin was able to bind to essential proteins for viral replication, such as nonstructural protein 1 (nsp-14) and Karyopherin-α1 (KPNA1), thus decreasing viral replication activity. Ivermectin also plays a vital role in several pro-inflammatory and anti-inflammatory cytokines, as inhibition of Toll-Like Receptors (TLRs), especially the TLR-4, blockade the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) transcriptional pathway, which might “protect” the host cell from the SARS-CoV-2 infection. IFN, interferon. *, Ivermectin is able to increase cell osmosis, and, in the figure, we exemplify its effect through the passage of Chloride (Cl). The figure was created in BioRender (BioRender.com).