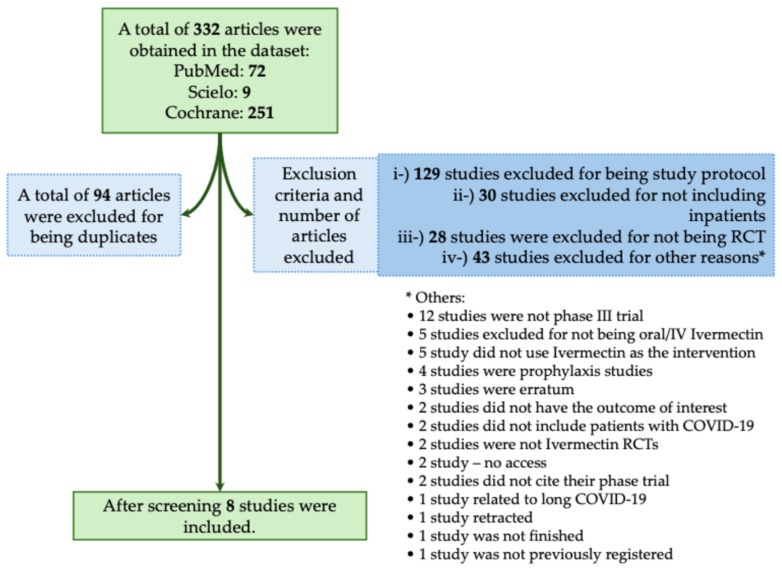
Figure 2.
Systematic review flowchart of clinical trials using Ivermectin during the coronavirus disease (COVID)-19 pandemic. We included in our systematic review a total of eight studies (Okumuş et al., 2021; Shakhsi Niaee et al., 2021; Beltran Gonzalez et al., 2022; Heydari et al., 2022; Lim et al., 2022; Qadeer et al., 2022; Rezai et al., 2022; Baghbanian et al., 2023) [17,18,19,20,21,22,23,24]. The data search was performed on PubMed-Medline, Cochrane, and SciELO from COVID-19 pandemic onset to December 2023. The following search was performed: Ivermectin: (((Ivermectin)) AND ((COVID-19) OR (COVID-19 treatments) OR (COVID-19 pandemic) OR (SARS-CoV-2) OR (SARS-CoV-2 infection))) AND (Therapy/Narrow[filter]) AND (randomized controlled trial[pt] OR controlled clinical trial[pt] OR clinical trials as topic[mesh:noexp] OR trial[ti] OR random*[tiab] OR placebo*[tiab]). RCT, randomized controlled trial; IV, intravenous. *, The 45 studies that were excluded from different criteria were presented separately due to the low number of studies per criteria.