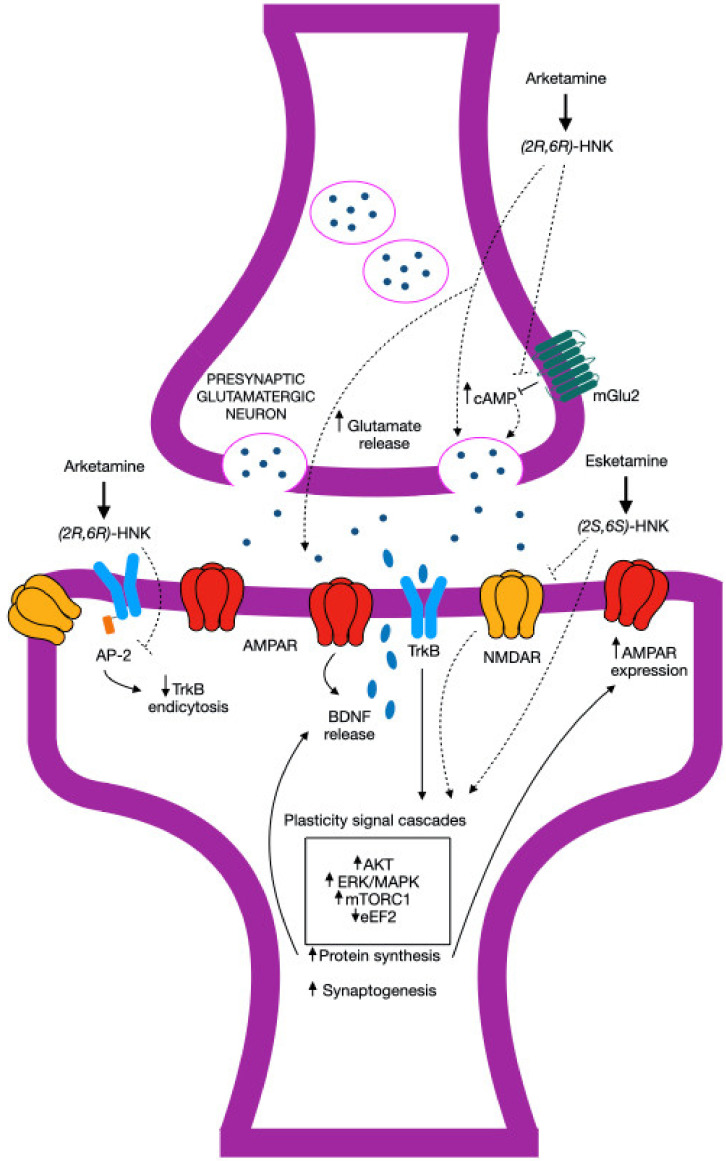
Figure 3.
Possible synaptic mechanisms of (2R,6R)- and (2S,6S)-hydroxynorketamine, according to [130]: (2R,6R)-HNK is believed to act on presynaptic terminals by increasing glutamate release, potentially through pathways that overlap with mGlu2 signaling. This may occur as (2R,6R)-HNK reduces the inhibition of cAMP release induced by mGlu2, or it may involve another mechanism driving glutamate release. The increased glutamate subsequently activates AMPA receptors (AMPAR), leading to the enhanced release of brain-derived neurotrophic factor (BDNF), activation of tropomyosin kinase B (TrkB) receptors, and the triggering of plasticity-related signaling pathways. These pathways include the upregulation of protein kinase B (AKT), extracellular signal-regulated kinases (ERK)/mitogen-activated protein kinases (MAPK), and mammalian/mechanistic target of rapamycin complex 1 (mTORC1), all of which promote protein synthesis, increase AMPAR expression, and support synapse formation, ultimately strengthening synaptic connections. Additionally, (2R,6R)-HNK may interfere with TrkB/AP-2 (Activator Protein-2) interactions, preventing TrkB endocytosis and stabilizing TrkB at the synapse. On the other hand, (2S,6S)-HNK moderately inhibits NMDA receptors (NMDARs) and might enhance intracellular signaling through an NMDAR inhibition-dependent mechanism, which includes the inhibition of eEF2 signaling, alongside increased AKT, ERK/MAPK, and mTORC1 activity.