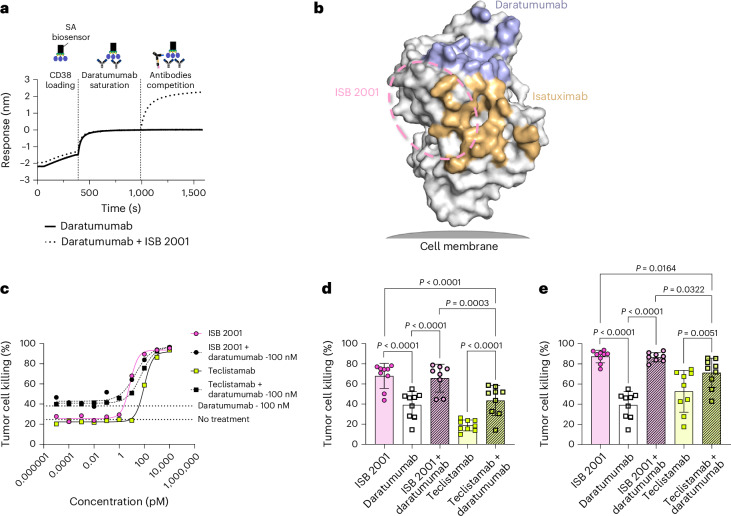
Fig. 5. ISB 2001 cytotoxic potency is not affected by daratumumab co-treatment and shows stronger killing potency than the combination of a BCMA TCE and daratumumab.
a, Competition binding assay by Octet BLI shows the absence of competition between ISB 2001 and daratumumab, as suggested by additive binding signal upon sequential exposure of a daratumumab-saturated CD38 sensor surface to ISB 2001 (dotted line). Saturation of the CD38 sensor surface was verified by dipping the daratumumab-saturated CD38 sensor surface into a solution of daratumumab at twofold concentration of saturation solution (solid line). Representative plot shows binding to the sensor tip as a wavelength shift (response) versus time (n = 2 independent measurements). b, Surface representation of CD38 illustrating the hypothetical epitope bin of ISB 2001 (dashed line), as determined from epitope binning assays including daratumumab and isatuximab. The epitopes of daratumumab (PDB 7DHA) and isatuximab (PDB 4CMH) are colored blue and orange, respectively. c, Cytotoxicity of the KMS-12-BM cell line at different concentrations of ISB 2001 and teclistamab in the presence or absence of 100 nM daratumumab in a MMoAK assay. Dotted lines show the no-treatment and daratumumab at 100 nM-only conditions. Four-parameter logistic curve (c) fitting from a representative donor (n = 9 individual PBMC donors and n = 8 individual donors for ISB 2001 + daratumumab, from n = 3 independent experiments). d,e, Cytotoxicity of ISB 2001 and teclistamab at 10 pM (d) or 100 pM (e) daratumumab (at 100 nM) and a combination of ISB 2001 or teclistamab (at 10 and 100 pM, respectively) plus daratumumab (at 100 nM) in a MMoAK assay. Each dot represents one individual donor (n = 9 or n = 8 for ISB 2001 + daratumumab) and bars show the mean ± s.d. from four independent experiments. Means were compared using a mixed-effects model followed by a Tukey’s multiple comparison and statistical differences are shown as exact P value when statistically significant (P < 0.05).