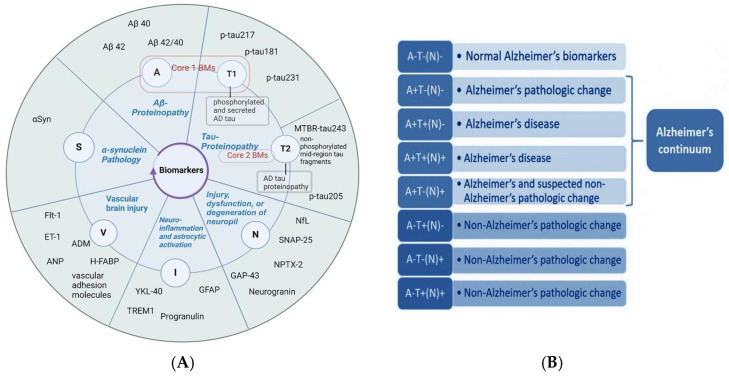
Figure 1.
(A): From ATN to AT1T2NIVS biomarker categorization of fluid analytes: The proposed new criteria by the NIA-AA 2024 working group emphasize ‘A’ and ‘T’ as the core biomarkers for the diagnosis and staging of AD. In addition, the revised scheme recognizes an expanded set of additional markers that detect non-specific biomarkers involved in AD pathophysiology (categorized under ‘N’ and ‘I’) and non-AD co-pathological biomarkers (categorized under ‘V’ and ‘S’). The core biomarkers are further divided into Core 1 and Core 2 biomarkers to reflect different stages of AD-related changes. (B): This figure depicts the biomarker profile and corresponding categorization based on the “A”, “T”, and “N” systems. By binarizing the three AT(N) biomarker types, eight distinct biomarker profiles are generated. Based on these profiles, individuals can be placed into one of three general categories: standard AD biomarkers, the Alzheimer’s continuum, or non-AD pathological changes.