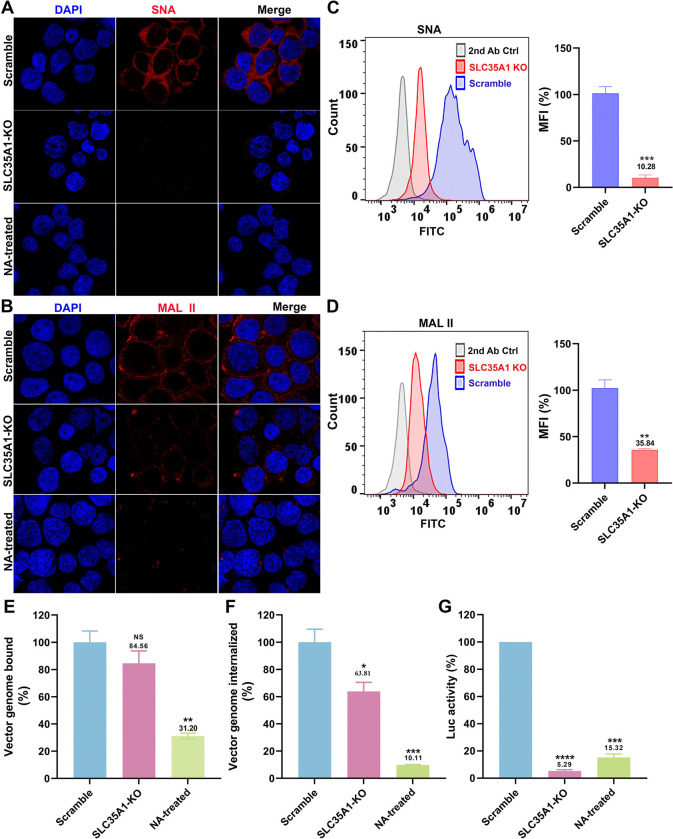
Figure 3. SLC35A1 KO significantly decreases SIA expression in HEK293SLC35A1-KO cells.
(A-F) Lectin staining. Biotinylated Sambucus Nigra lectin (SNA) and Maackia Amurensis lectin II (MAL II) lectins were used to stain glycan expression in HEK293SLC35A1-KO cells. NA-treated cells served as a positive control to show the removal of sialic acids. (A&B) Confocal microscopy. SNA (A) and MAL II (B) stained cells were incubated with DyLight 649-conjugated streptavidin for visualization at 100 × under a confocal microscope (Leica SP8 STED). (C&D) Flow cytometry. (C) SNA and (D) MAL II stained cells were incubated with FITC-conjugated streptavidin for flow cytometry. The histograms show the intensity of the FITC staining on the x-axis and the number of cells at each intensity level on the y-axis. The mean fluorescence intensity (MFI) values were calculated, normalized to the wild-type (WT) HEK293 cells as percentages (%), and are shown with a mean and SD from three replicates. P values were determined by using the Student’s t-test. (E-G) rAAV5 vector transduction, binding, and internalization in HEK293 cells. Relative percentages of vector binding (E), internalization (F), and transduction (G) to the Scramble cell group are calculated in rAAV-transduced SLC35A1-KO or NA-treated scramble HEK293 cells. The data shown were a mean and SD from three replicates. P values were determined by using one-way ANOVA for the comparison of the vector value in the KO or NA-treated cell group and the scramble cell group.