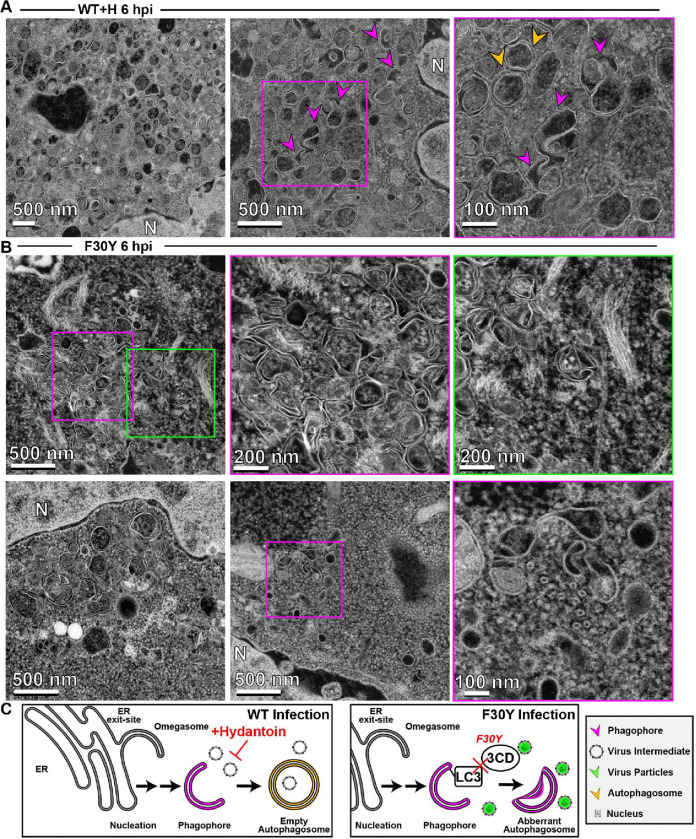
Figure 8. 3CD-mutant PV exhibits defects to autophagosome biogenesis and virion loading.
(A) HAADF-STEM imaging of WT PV-infected HeLa cells in the presence of hydantoin. HeLa cells were infected with WT PV at an MOI of 10 in the presence of 50 μg/mL hydantoin and then fixed in glutaraldehyde at the indicated time points. Fixed samples were dehydrated, stained, embedded, and sectioned in thin micrographs for imaging as described (Fig. S1). Arrows indicate observed structures. Phagophore (magenta), virus particles (green), autophagosomes (yellow), and nucleus (N). Hydantoin impairs virus assembly, as evidenced by the lack of virus particles observed in the image. Omegasomes, empty double-membrane vesicles (DMVs), and fiber-like structure-containing DMVs are abundant in these samples. Intra-luminal vesicles in amphisome-like vesicles appear empty. These ultrastructural changes are observed both at 6 and 8 hpi. (B) HAADF-STEM imaging of F30Y PV-infected HeLa cells. HeLa cells were infected with F30Y PV at an MOI of 10 and then fixed in glutaraldehyde at the indicated time points. Fixed samples were dehydrated, stained, embedded, and sectioned in thin micrographs for imaging as described (Fig. S1). Arrows indicate observed structures. Phagophore (magenta), virus particles (green), autophagosomes (yellow), and nucleus (N). F30Y interferes with DMV maturations with an exaggerated amount of omegasomes and aberrant DMVs observed by 6 hpi. Few virions are observed, some of which appear “stuck” in an omegasome. (C) Autophagic signals during F30Y PV infection. An ER-derived omegasome buds out and is engaged by multiple autophagy-associated proteins, adaptors, kinases, and protein complexes to yield an autophagophore in preparation for virion loading and maturation of a double-membranous vesicle termed autophagosome. This step is blocked by hydantoin. Autophagosome maturation is triggered by the lipidated form of the essential microtubule-associated protein 1A/1B-light chain 3 (LC3) protein. Virus is recruited to the phagophore by combining factors, including LC3 and 3CD. F30Y 3CD interferes with this step.