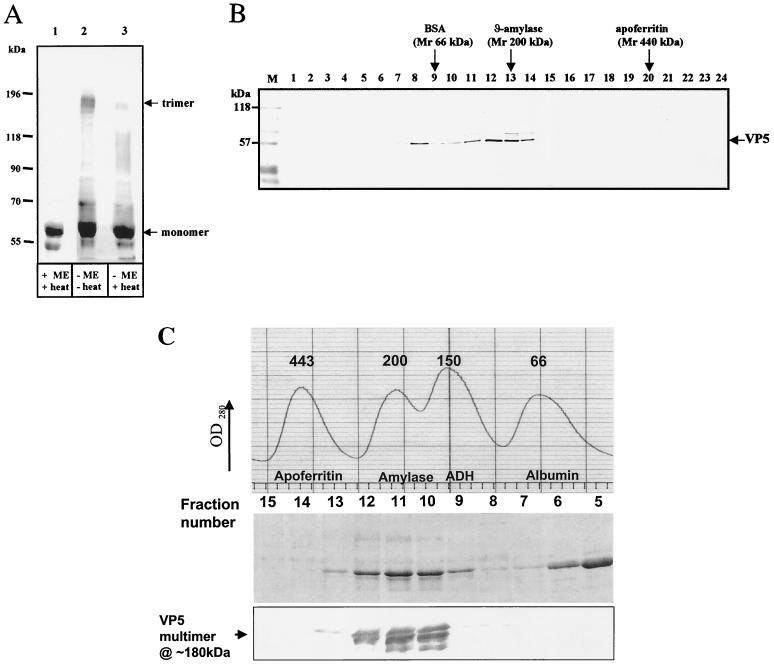
FIG. 8.
Identification of VP5 oligomers. (A) Western blot showing VP5 oligomers in reducing and nonreducing conditions. Purified soluble VP5 was analyzed by SDS–7% PAGE, blotted onto nitrocellulose paper, and probed with an anti-VP5 PAb. The VP5 were investigated in the presence (+ME) or absence (−ME) of 1% β-mercaptoethanol and with (+heat) or without (−heat) heating to 100°C for 4 min. The samples were resuspended in sample buffer containing 1% SDS, 15% glycerol, and 10 mM Tris-HCl (pH 6.8). The trimer (180 kDa) and monomer (∼59 kDa) are indicated. Additional smaller bands are the VP5 degraded products. (B) Western blot of purified VP5 after fractionation by glycerol gradient (20 to 40%) centrifugation. Lanes 1 to 24 represent fractions collected from the glycerol gradient; fraction 1 represents the top of the gradient, and fraction 24 represents the bottom. After SDS–10 PAGE, the gels were blotted onto nitrocellulose paper and probed with an anti-VP5 PAb. The positions of the standard size marker proteins of BSA (66 kDa), β-amylase (200 kDa), and apoferritin (440 kDa) are indicated at the top. The standard size markers were run in parallel in different tubes than the samples but in equivalent glycerol gradient fractions. The prestained markers on the blot are designated M. (C) Size exclusion chromatography of purified VP5. Recombinant VP5 expressed in insect cells was partially purified by anion-exchange and hydrophobic-interaction-exchange chromatography. VP5 was applied to a Superdex-200 column previously calibrated with a standard set of marker proteins (the profile is shown in the uppermost panel). VP5 fractions 5 to 13 covering the molecular mass range from 66 to 400 kDa were analyzed by SDS–10% PAGE (middle panel), and fractions 8 to 13 only were analyzed by Western blot with polyclonal VP5 antiserum (lower panel). The identity of the high-molecular-mass VP5 species as the putative trimer is indicated.