Figure 8.
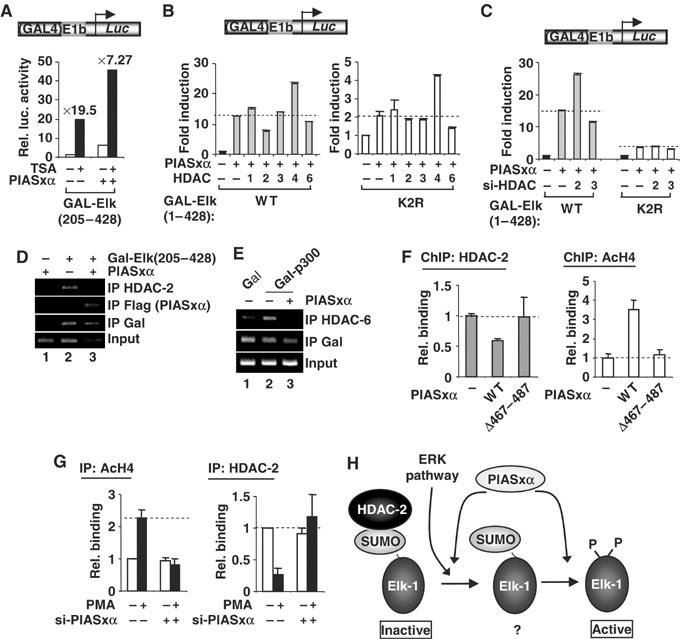
PIASxα affects HDAC-2 binding and histone acetylation levels at Elk-1-regulated promoters. (A–C) Reporter gene analysis of the activities of GAL-Elk-1 constructs on a GAL-driven E1B promoter-reporter construct in 293 cells. (A) The activity of GAL-Elk(205–428) in the presence or absence of PIASxα and/or TSA. Data are shown as luciferase assays relative to GAL-Elk(205–428) alone and as fold induction by TSA. (B) The activities of wild-type (WT) and mutant (K2R) GAL-Elk(1–428) in the presence and absence of PIASxα and/or indicated HDACs. Data are shown as fold induction to the reporter activity relative to each of GAL fusion proteins in the absence of PIASxα (taken as 1). Note the different scales on the axes for WT and K2R versions of Elk-1. (C) The activities of GAL-Elk(1–428) WT and K2R in the presence or absence of RNAi duplexes against GAPDH (−) or the indicated HDACs. Data are shown as fold induction to the reporter activity relative to each of GAL fusion proteins in the presence of GAPDH RNAi duplexes. (D, E) ChIP analysis of HDAC-2, PIASxα (D) and HDAC-6 (E) recruitment to a GAL-driven E1b promoter-reporter construct in 293 cells. The recruitment is monitored following immunoprecipitation (IP) with the indicated antibodies in the presence of the indicated combinations of Gal4 DBD, Gal-Elk(205–428) or Gal-p300 and PIASxα. (F, G) ChIP analysis of HDAC-2 and acetyl-H4 levels on the c-fos promoter. (F) Assays were carried out in 293 cells in the presence of transfected Elk-1 and in the presence or absence of the indicated PIASxα proteins using antisera specific for the indicated proteins. Following immunoprecipitation (IP) of crosslinked lysates, real-time PCR analysis of eluted DNA was performed. All quantitative PCRs were normalised to the input control. Data are shown as relative enrichment for each individual antibody used. (G) ChIP analysis of HeLa cells containing transfected RNAi duplexes against PIASxα using antisera specific for the indicated proteins. (H) Model for the action of PIASxα in facilitating HDAC-2 removal from sumoylated Elk-1. The ERK pathway causes HDAC-2 release and Elk-1 phosphorylation and desumoylation. PIASxα promotes HDAC-2 release and is required for desumoylation. A poised ‘intermediate' sumoylated form of Elk-1 (indicated by ‘?'), which precedes the appearance of a phosphorylated, desumoylated fully active form, is indicated to reflect the molecular role of PIASxα.
