Figure 3.
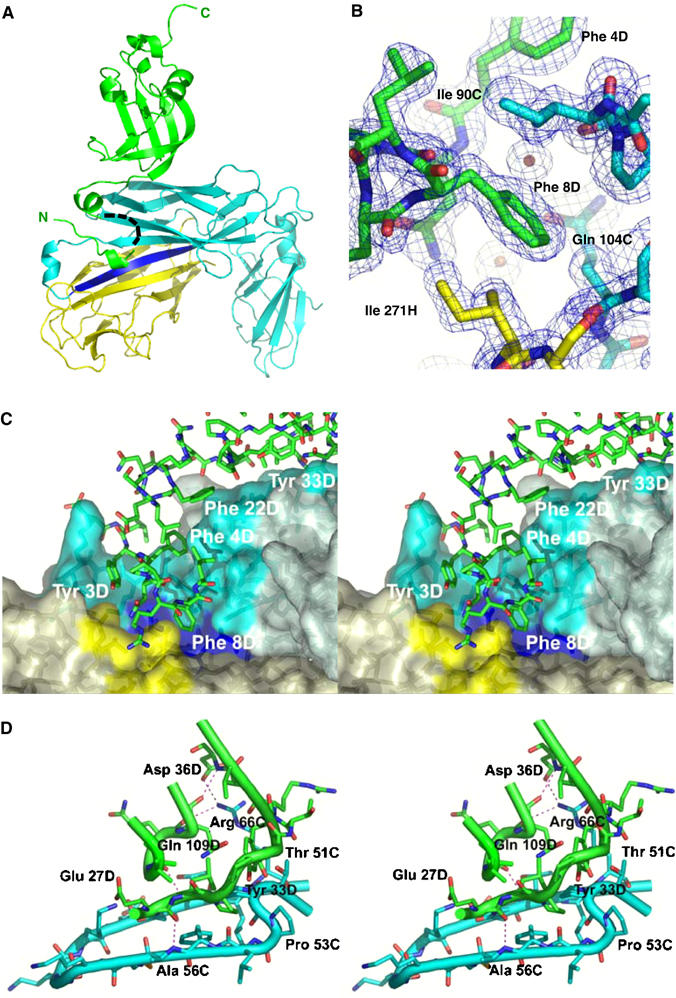
X-ray structure of the ternary FimDN(1–125)–FimC–FimHP complex. (A) Ribbon diagram of the ternary complex, with FimDN(1–125) depicted in green, FimC in cyan and the pilin domain FimHP in yellow. The G1 donor strand of FimC is colored in blue. A black dashed line indicates residues 10–18 of FimDN, for which no electron density was observed. The N- and C-termini of FimDN are labeled in green. (B) Close-up view of the hydrophobic contacts between Phe8 of the N-terminal FimDN tail (green) and residues from FimC (cyan) and FimHP (yellow). The final 2mFo−DFc electron density map is contoured at 1σ level. (C) Stereo representation of the tail interface. Residues from FimDN, in stick model, are shown in green. The molecular surfaces of FimC (slate-grey) and FimHP (light yellow) are shown in semitransparent mode. Residues contributing to the FimC and FimHP surfaces and interacting with FimDN are shown in more intense color: cyan for FimC and yellow for FimHP residues, respectively. Residues from the G1 donor strand of FimC contributing to the molecular surface appear in blue. (D) Stereo representation of the interface between FimC and the folded FimDN core 25–125. Some hydrogen bonds between FimC and the FimDN core are depicted as thin dashed lines. Color coding is as in (A). The figure was prepared with Pymol (www.pymol.org).
