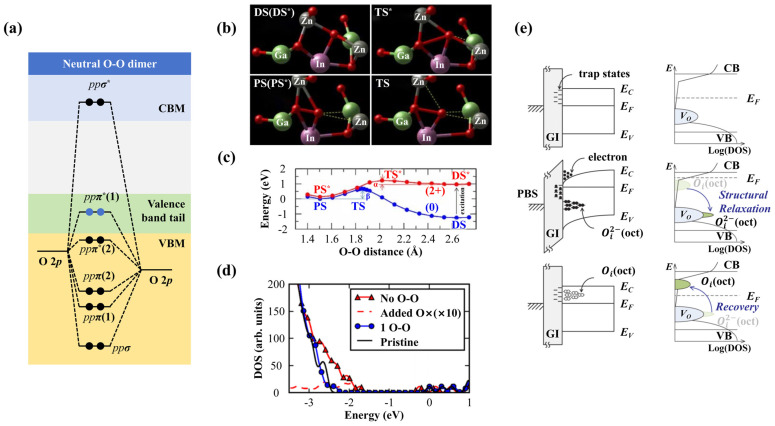
Figure 5.
(a) Schematic illustration of the orbital energy levels of a neutral O-O bond. The blue circles represent the pair of electrons donated by adjacent metal ions. (b) Local atomic configurations of the O-O bonds formed in a-InGaZnO4. These configurations include the disordered state (DS), transition state (TS), and peroxide state (PS) in the neutral condition, along with their corresponding +2 charged states DS*, TS*, and PS*. (c) Energy barriers associated with the formation and dissociation of the O-O bond calculated by DFT in both neutral (blue curve) and charged (red curve) states. (d) DOS for a-InGaZnO4 under different conditions. The O-O bond is formed by the introduction of Oi. (e) Band structure diagrams showing the instability caused by Oi under PBS. (a–d) Reprinted from [38,49], Copyright (2015, 2018) by American Physical Society. (b,c) Reprinted from [50], Copyright (2012) by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (e) Reprinted from [51], Copyright (2017) by IEEE.