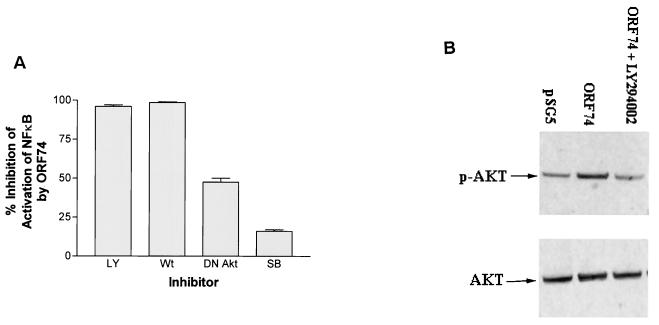
FIG. 8.
Involvement of PI 3-kinase and Akt but not p38 in NF-κB activation by ORF74. (A) Inhibition of NF-κB activation. KSIMM cells (106) were transfected with 0.3 μg of pSG5-ORF74 and 0.5 μg of the NF-κB luciferase reporter plasmid. Cells were treated with the PI 3-kinase inhibitors LY294002 (LY) (40 μM) and wortmannin (Wt) (20 nM) or by the p38 inhibitor SB203580 (SB) (10 μM) in dimethyl sulfoxide (DMSO) for 12 h and then assayed for luciferase activity as described in Materials and Methods. Controls were treated with DMSO alone. Alternatively, cells were cotransfected with ORF74-pSG5 and 0.2 μg of DN Akt mutant expression construct or a control plasmid and then assayed for luciferase activity as described above. All samples were analyzed in triplicate, and the values were averaged. Data are presented as the percent inhibition of NF-κB activity induced by ORF74 as assayed by measuring luciferase activity. (B) Akt phosphorylation. Cell lysates from KSIMM cells transfected with pSG5 or ORF74-pSG5 were subjected to SDS-PAGE and Western blot analysis using an antibody specific for phosphorylated Akt (p-AKT). Lysates from cells transfected with ORF74 and treated with the PI 3-kinase inhibitor LY294002 (40 μM) were also analyzed. Blots were stripped and reprobed with an antibody to total Akt (AKT) to determine the relative phosphorylation levels. The transfection efficiencies were ca. 25%.