Abstract
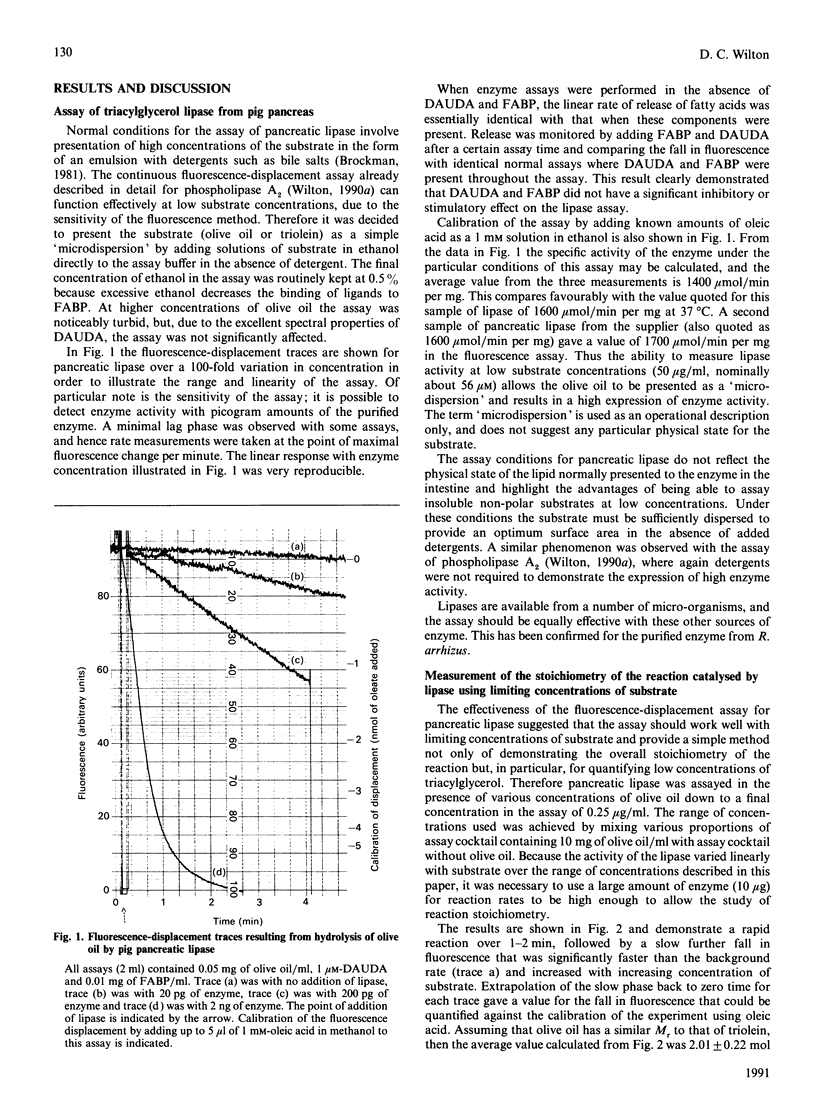
A new continuous fluorescence-displacement assay for enzymes that release long-chain fatty acids [Wilton (1990) Biochem. J. 266, 435-439] is described in detail for pig pancreatic triacylglycerol lipase. The assay involves the displacement of the highly fluorescent fatty acid probe 11-(dansylamino)undecanoic acid from rat liver fatty acid-binding protein by long-chain fatty acids released as a result of enzyme activity. The assay is surprisingly effective for triacylglycerol lipase, allowing the expression of full activity with low concentrations of substrates in the absence of detergents. The initial rate of decrease in fluorescence is linearly related to enzyme concentration, and activity can be detected in the assay down to concentrations of 10 pg of pure enzyme/ml. The assays demonstrated the quantitative conversion of limiting amounts of substrate into the monacylglycerol. This observation allowed the assay to be used to measure substrates such as triacylglycerols and particularly 1,2-diacylglycerols at concentrations down to about 0.1 microM. Because phospholipase C releases 1,2-diacylglycerols, the coupling of this enzyme to excess lipase allowed the measurement of pure phospholipase C from Bacillus cereus at concentrations down to about 2 ng/ml, and the initial rate of fall in fluorescence in the assay was linearly related to enzyme activity.
Full text
PDF
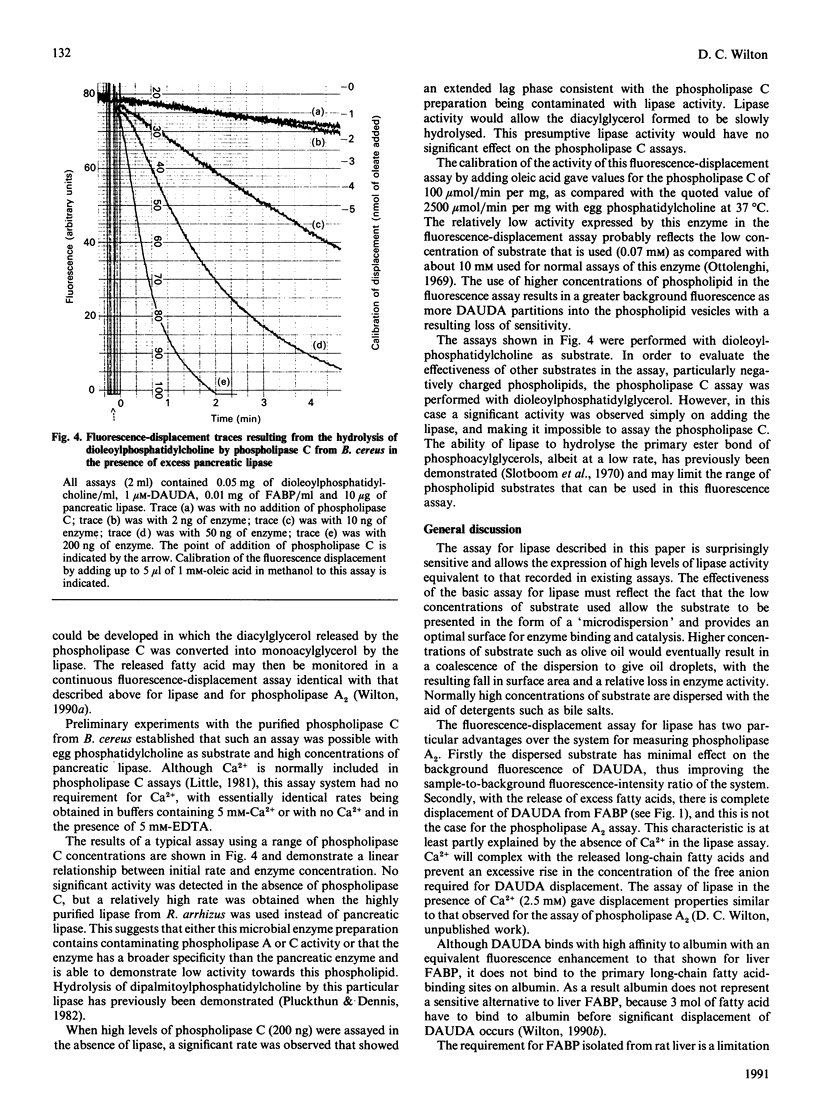

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brady L., Brzozowski A. M., Derewenda Z. S., Dodson E., Dodson G., Tolley S., Turkenburg J. P., Christiansen L., Huge-Jensen B., Norskov L. A serine protease triad forms the catalytic centre of a triacylglycerol lipase. Nature. 1990 Feb 22;343(6260):767–770. doi: 10.1038/343767a0. [DOI] [PubMed] [Google Scholar]
- Brockman H. L. Triglyceride lipase from porcine pancreas. Methods Enzymol. 1981;71(Pt 100):619–627. doi: 10.1016/0076-6879(81)71074-7. [DOI] [PubMed] [Google Scholar]
- Plückthun A., Dennis E. A. Acyl and phosphoryl migration in lysophospholipids: importance in phospholipid synthesis and phospholipase specificity. Biochemistry. 1982 Apr 13;21(8):1743–1750. doi: 10.1021/bi00537a007. [DOI] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S. Production of diglyceride from phosphatidylinositol in activated human platelets. J Clin Invest. 1979 Apr;63(4):580–587. doi: 10.1172/JCI109339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson T. C., Wilton D. C. Studies on fatty acid-binding proteins. The binding properties of rat liver fatty acid-binding protein. Biochem J. 1987 Oct 15;247(2):485–488. doi: 10.1042/bj2470485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson T. C., Wilton D. C. Studies on fatty acid-binding proteins. The detection and quantification of the protein from rat liver by using a fluorescent fatty acid analogue. Biochem J. 1986 Sep 1;238(2):419–424. doi: 10.1042/bj2380419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton D. C. A continuous fluorescence displacement assay for the measurement of phospholipase A2 and other lipases that release long-chain fatty acids. Biochem J. 1990 Mar 1;266(2):435–439. doi: 10.1042/bj2660435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton D. C. Studies on fatty-acid-binding proteins. The purification of rat liver fatty-acid-binding protein and the role of cysteine-69 in fatty acid binding. Biochem J. 1989 Jul 1;261(1):273–276. doi: 10.1042/bj2610273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton D. C. The fatty acid analogue 11-(dansylamino)undecanoic acid is a fluorescent probe for the bilirubin-binding sites of albumin and not for the high-affinity fatty acid-binding sites. Biochem J. 1990 Aug 15;270(1):163–166. doi: 10.1042/bj2700163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler F. K., D'Arcy A., Hunziker W. Structure of human pancreatic lipase. Nature. 1990 Feb 22;343(6260):771–774. doi: 10.1038/343771a0. [DOI] [PubMed] [Google Scholar]


